Humoral Response to BNT162b2 Vaccine Against SARS-CoV-2 Variants Decays After Six Months
- PMID: 35585980
- PMCID: PMC9108166
- DOI: 10.3389/fimmu.2022.879036
Humoral Response to BNT162b2 Vaccine Against SARS-CoV-2 Variants Decays After Six Months
Abstract
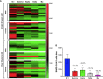
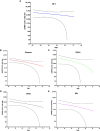
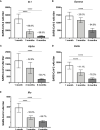
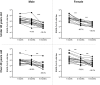
SARS-CoV-2 vaccines have shown very high effectiveness in real-world scenarios. However, there is compelling evidence for a fast-paced waning of immunity. The increasing number of new variants that could alter the severity, transmissibility, and potential to evade the immune response raised significant concern. Therefore, elucidating changes in the humoral immune response against viral variants induced by vaccines over time is crucial for improving immunization protocols. We carried out a 6-month longitudinal prospective study in which 60 individuals between 21 and 71 years of age who have received the complete scheme of the BNT162b2 vaccine were followed to determine titers of serum neutralizing activity. The neutralizing capacity was measured at one, three, and six-months post-vaccination by plaque reduction neutralization assay using SARS-CoV-2 B.1 (D614G) and the Gamma, Alpha, Delta, and Mu variants. Data were analyzed using GraphPad 5.0. Neutralizing activity against five different SARS-CoV-2 variants was detected in the serum samples of all vaccinated participants to a different extent after one month, with a progressive decrease according to age and gender. Overall, after one month of vaccination, the neutralizing titer was lower for all evaluated variants when compared to B.1, most remarkable against Delta and Mu, with a reduction of 83.1% and 92.3%, respectively. In addition, the Titer at 3- or 6-months follow-up decreased dramatically for all variants. Our results support the decaying of serum neutralizing activity, both over time and across SARS-CoV-2 variants, being more significant in older men. Since Delta and Mu appear to evade the neutralizing activity, these and further new variants of immune escape mutations should be considered for novel vaccine formulations.
Keywords: SARS-CoV-2; immunity; immunogenicity; neutralizing antibodies; vaccine; variant.
Copyright © 2022 Lopera, Chvatal-Medina, Flórez-Álvarez, Zapata-Cardona, Taborda, Rugeles and Hernandez.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Johns Hopkins University & Medicine . COVID-19 Map - Johns Hopkins Coronavirus Resource Center. Johns Hopkins Coronavirus Resour Cent, Baltimore, Maryland; (2020).
-
- Tartof SY, Slezak JM, Fischer H, Hong V, Ackerson BK, Ranasinghe ON, et al. Effectiveness of mRNA BNT162b2 COVID-19 Vaccine Up to 6 Months in a Large Integrated Health System in the USA: A Retrospective Cohort Study. Lancet (London England) (2021) 398:1407–16. doi: 10.1016/S0140-6736(21)02183-8 - DOI - PMC - PubMed
-
- World Health Organization . Tracking SARS-CoV-2 Variants. WHO, Ginebra, Swizertland, (2021). Available at: https://www.who.int/en/activities/tracking-SARS-Co.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

