T Lymphocyte Exhaustion During Human and Experimental Visceral Leishmaniasis
- PMID: 35585983
- PMCID: PMC9108272
- DOI: 10.3389/fimmu.2022.835711
T Lymphocyte Exhaustion During Human and Experimental Visceral Leishmaniasis
Abstract
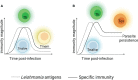
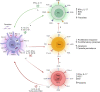
A key point of immunity against protozoan Leishmania parasites is the development of an optimal T cell response, which includes a low apoptotic rate, high proliferative activity and polyfunctionality. During acute infection, antigen-specific T cells recognize the pathogen resulting in pathogen control but not elimination, promoting the development and the maintenance of a population of circulating effector cells that mount rapid response quickly after re-exposure to the parasite. However, in the case of visceral disease, the functionality of specific T cells is lost during chronic infection, resulting in inferior effector functions, poor response to specific restimulation, and suboptimal homeostatic proliferation, a term referred to as T cell exhaustion. Multiple factors, including parasite load, infection duration and host immunity, affect T lymphocyte exhaustion. These factors contribute to antigen persistence by promoting inhibitory receptor expression and sustained production of soluble mediators, influencing suppressive cell function and the release of endogenous molecules into chronically inflamed tissue. Together, these signals encourage several changes, reprogramming cells into a quiescent state, which reflects disease progression to more severe forms, and development of acquired resistance to conventional drugs to treat the disease. These points are discussed in this review.
Keywords: T cell exhaustion; Th subsets; inflammation; inhibitory receptor; visceral leishmanaisis.
Copyright © 2022 Costa-Madeira, Trindade, Almeida, Silva and Carregaro.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
T cell suppression in the bone marrow of visceral leishmaniasis patients: impact of parasite load.Clin Exp Immunol. 2018 Mar;191(3):318-327. doi: 10.1111/cei.13074. Epub 2017 Nov 20. Clin Exp Immunol. 2018. PMID: 29058314 Free PMC article.
-
Programmed death 1-mediated T cell exhaustion during visceral leishmaniasis impairs phagocyte function.J Immunol. 2013 Dec 1;191(11):5542-50. doi: 10.4049/jimmunol.1301810. Epub 2013 Oct 23. J Immunol. 2013. PMID: 24154626 Free PMC article.
-
Leishmania infantum antigens modulate memory cell subsets of liver resident T lymphocyte.Immunobiology. 2017 Feb;222(2):409-422. doi: 10.1016/j.imbio.2016.08.009. Epub 2016 Sep 1. Immunobiology. 2017. PMID: 27615509
-
Cytokine saga in visceral leishmaniasis.Cytokine. 2021 Nov;147:155322. doi: 10.1016/j.cyto.2020.155322. Epub 2020 Oct 28. Cytokine. 2021. PMID: 33127259 Review.
-
The Paradox of a Phagosomal Lifestyle: How Innate Host Cell-Leishmania amazonensis Interactions Lead to a Progressive Chronic Disease.Front Immunol. 2021 Sep 7;12:728848. doi: 10.3389/fimmu.2021.728848. eCollection 2021. Front Immunol. 2021. PMID: 34557194 Free PMC article. Review.
Cited by
-
CXCR5 and TIM-3 expressions define distinct exhausted T cell subsets in experimental cutaneous infection with Leishmania mexicana.Front Immunol. 2023 Aug 25;14:1231836. doi: 10.3389/fimmu.2023.1231836. eCollection 2023. Front Immunol. 2023. PMID: 37691941 Free PMC article.
-
CD4+ Th1 and Th17 responses and multifunctional CD8 T lymphocytes associated with cure or disease worsening in human visceral leishmaniasis.Front Immunol. 2024 Feb 12;15:1277557. doi: 10.3389/fimmu.2024.1277557. eCollection 2024. Front Immunol. 2024. PMID: 38410517 Free PMC article.
-
An integrated analysis of the structural changes and gene expression of spleen in human visceral leishmaniasis with and without HIV coinfection.PLoS Negl Trop Dis. 2024 Jun 6;18(6):e0011877. doi: 10.1371/journal.pntd.0011877. eCollection 2024 Jun. PLoS Negl Trop Dis. 2024. PMID: 38843306 Free PMC article.
-
Within-host bayesian joint modeling of longitudinal and time-to-event data of Leishmania infection.PLoS One. 2024 Feb 9;19(2):e0297175. doi: 10.1371/journal.pone.0297175. eCollection 2024. PLoS One. 2024. PMID: 38335163 Free PMC article.
-
Chemokines Signature and T Cell Dynamics in Leishmaniasis: Molecular insight and therapeutic application.Expert Rev Mol Med. 2024 Nov 26;27:1-55. doi: 10.1017/erm.2024.36. Online ahead of print. Expert Rev Mol Med. 2024. PMID: 39587036 Free PMC article. Review.
References
-
- dos Santos Marques LH, Rocha DAIC, Reis IA, Cunha DAGM, Oliveira E, Pfeilsticker TR, et al. . Leishmania Infantum: Illness, Transmission Profile and Risk Factors for Asymptomatic Infection in an Endemic Metropolis in Brazil. Parasitol (2017) 144(4):546–56. doi: 10.1017/S0031182016002134 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

