Hepatoprotective Effect of Oplopanax elatus Nakai Adventitious Roots Extract by Regulating CYP450 and PPAR Signaling Pathway
- PMID: 35586046
- PMCID: PMC9108204
- DOI: 10.3389/fphar.2022.761618
Hepatoprotective Effect of Oplopanax elatus Nakai Adventitious Roots Extract by Regulating CYP450 and PPAR Signaling Pathway
Abstract
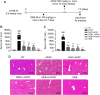
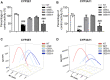
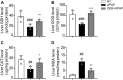
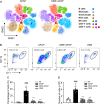
O. elatus Nakai is a traditional medicine that has been confirmed to exert effective antioxidant and anti-inflammatory functions, and is used for the treatment of different disorders. However, its potential beneficial effects on drug induced hepatotoxicity and relevant molecular mechanisms remain unclear. This study investigated the protective effect and further elucidated the mechanisms of action of O. elatus on liver protection. O. elatus chlorogenic acids-enriched fraction (OEB), which included chlorogenic acid and isochlorogenic acid A, were identified by HPLC-MS/MS. OEB was administrated orally daily for seven consecutive days, followed by a single intraperitoneal injection of an overdose of APAP after the final OEB administration. The effects of OEB on immune cells in mice liver were analyzed using flow cytometry. APAP metabolite content in serum was detected using HPLC-MS/MS in order to investigate whether OEB affects CYP450 activities. The intestinal content samples were processed for 16 s microbiota sequencing. Results demonstrated that OEB decreased alanine aminotransferase, aspartate aminotransferase contents, affected the metabolism of APAP, and decreased the concentrates of APAP, APAP-CYS and APAP-NAC by inhibiting CYP2E1 and CYP3A11 activity. Furthermore, OEB pretreatment regulated lipid metabolism by affecting the peroxisome proliferator-activated receptors (PPAR) signaling pathway in mice and also increased the abundance of Akkermansia and Parabacteroides. This study indicated that OEB is a potential drug candidate for treating hepatotoxicity because of its ability to affect drug metabolism and regulate lipid metabolism.
Keywords: CYP450; Oplopanax elatus Nakai; PPAR; adventitious roots; gut microbiota; neutrophil.
Copyright © 2022 Jiang, Luo, Zhou, Luo, Hao, Fan, Wu, Gao, Bi, Zhao, Lian and Lian.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Adventitious root cultures of Oplopanax elatus inhibit LPS-induced inflammation via suppressing MAPK and NF-κB signaling pathways.In Vitro Cell Dev Biol Anim. 2019 Oct;55(9):766-775. doi: 10.1007/s11626-019-00396-7. Epub 2019 Sep 16. In Vitro Cell Dev Biol Anim. 2019. PMID: 31529418
-
Chemo-Preventive Potential of Falcarindiol-Enriched Fraction from Oplopanax elatus on Colorectal Cancer Interfered by Human Gut Microbiota.Am J Chin Med. 2019;47(6):1381-1404. doi: 10.1142/S0192415X1950071X. Epub 2019 Sep 5. Am J Chin Med. 2019. PMID: 31488036
-
Chemical profiling of root bark extract from Oplopanax elatus and its in vitro biotransformation by human intestinal microbiota.PeerJ. 2021 Nov 24;9:e12513. doi: 10.7717/peerj.12513. eCollection 2021. PeerJ. 2021. PMID: 34900430 Free PMC article.
-
Oplopanax elatus (Nakai) Nakai: chemistry, traditional use and pharmacology.Chin J Nat Med. 2014 Oct;12(10):721-9. doi: 10.1016/S1875-5364(14)60111-4. Epub 2014 Oct 31. Chin J Nat Med. 2014. PMID: 25443364 Review.
-
The phenolic acids from Oplopanax elatus Nakai stems and their potential photo-damage prevention activity.J Nat Med. 2022 Jan;76(1):39-48. doi: 10.1007/s11418-021-01546-6. Epub 2021 Aug 3. J Nat Med. 2022. PMID: 34345982
Cited by
-
Polyphenols as potential metabolism mechanisms regulators in liver protection and liver cancer prevention.Cell Prolif. 2023 Jan;56(1):e13346. doi: 10.1111/cpr.13346. Epub 2022 Oct 13. Cell Prolif. 2023. PMID: 36229407 Free PMC article. Review.
References
-
- Bae H. R., Leung P. S. C., Hodge D. L., Fenimore J. M., Jeon S-M., Thovarai V., et al. (2020). Multi-Omics: Differential Expression of IFN-γ Results in Distinctive Mechanistic Features Linking Chronic Inflammation, Gut Dysbiosis, and Autoimmune Diseases - ScienceDirect. J. Autoimmun. 111, 102436. 10.1016/j.jaut.2020.102436 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

