DNAJA1 Stabilizes EF1A1 to Promote Cell Proliferation and Metastasis of Liver Cancer Mediated by miR-205-5p
- PMID: 35586205
- PMCID: PMC9110222
- DOI: 10.1155/2022/2292481
DNAJA1 Stabilizes EF1A1 to Promote Cell Proliferation and Metastasis of Liver Cancer Mediated by miR-205-5p
Abstract
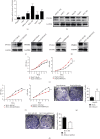
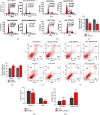
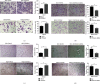
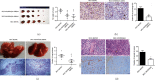
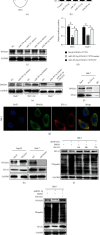
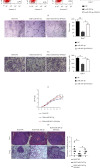
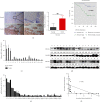
Liver cancer is one of the most common and aggressive malignancies worldwide with poor prognosis. Studies on pathogenesis of liver cancer are urgently demanded to develop better treatment strategy. Here, we found that overexpression of DnaJ heat shock protein family (Hsp40) member A1 (DNAJA1) increased cell proliferation, invasion, and angiogenesis in Huh 7 and HepG2 cells, while depletion of DNAJA1 in MHCC-97H and HCC-M3 showed opposite effects. In vivo functional assays indicated that DNAJA1 promoted tumor growth and pulmonary metastasis in mice. Mechanistically, as a direct target of miR-205-5p, DNAJA1 promoted proliferation and metastasis of liver cancer cells by stabilizing eukaryotic elongation factor 1A1 (EF1A1). Moreover, DNAJA was markedly upregulated in liver cancer tissues (P < 0.05) and was significantly associated with poor prognosis. And its expression was correlated with differentiation (P < 0.001), dissemination (P < 0.001), and serum AFP (P = 0.029). The mRNA levels of miR-205-5p and DNAJA1 were negatively correlated in liver cancer. In conclusion, our study reveals that DNAJA1 acts as an oncogene in liver cancer via miR-205-5p/EF1A1 axis and might be a potential biomarker to predict the prognosis for liver cancer patients.
Copyright © 2022 Lizhi Yi et al.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
References
-
- Bosch F. X., Ribes J., Díaz M., Cléries R. Gastroenterology (New York, N.Y. 1943) Vol. 127. United States: Elsevier BV; 2004. Primary liver cancer: eorldwide incidence and trends; pp. S5–S16. - PubMed
LinkOut - more resources
Full Text Sources

