Mycobacterium tuberculosis Induces Irg1 in Murine Macrophages by a Pathway Involving Both TLR-2 and STING/IFNAR Signaling and Requiring Bacterial Phagocytosis
- PMID: 35586249
- PMCID: PMC9109611
- DOI: 10.3389/fcimb.2022.862582
Mycobacterium tuberculosis Induces Irg1 in Murine Macrophages by a Pathway Involving Both TLR-2 and STING/IFNAR Signaling and Requiring Bacterial Phagocytosis
Abstract
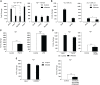
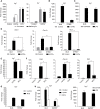
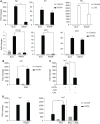
Irg1 is an enzyme that generates itaconate, a metabolite that plays a key role in the regulation of inflammatory responses. Previous studies have implicated Irg1 as an important mediator in preventing excessive inflammation and tissue damage in Mycobacterium tuberculosis (Mtb) infection. Here, we investigated the pattern recognition receptors and signaling pathways by which Mtb triggers Irg1 gene expression by comparing the responses of control and genetically deficient BMDMs. Using this approach, we demonstrated partial roles for TLR-2 (but not TLR-4 or -9), MyD88 and NFκB signaling in Irg1 induction by Mtb bacilli. In addition, drug inhibition studies revealed major requirements for phagocytosis and endosomal acidification in Irg1 expression triggered by Mtb but not LPS or PAM3CSK4. Importantly, the Mtb-induced Irg1 response was highly dependent on the presence of the bacterial ESX-1 secretion system, as well as host STING and Type I IFN receptor (IFNAR) signaling with Type II IFN (IFN-γ) signaling playing only a minimal role. Based on these findings we hypothesize that Mtb induces Irg1 expression in macrophages via the combination of two independent triggers both dependent on bacterial phagocytosis: 1) a major signal stimulated by phagocytized Mtb products released by an ESX-1-dependent mechanism into the cytosol where they activate the STING pathway leading to Type I-IFN production, and 2) a secondary TLR-2, MyD88 and NFκB dependent signal that enhances Irg1 production independently of Type I IFN induction.
Keywords: ESAT-6; ESX-1 system; IFN; Irg1; Mycobacterium tuberculosis; STING; TLR-2; macrophages.
Copyright © 2022 Bomfim, Fisher, Amaral, Mittereder, McCann, Correa, Namasivayam, Swamydas, Moayeri, Weiss, Chari, McVicar, Costa, D’Império Lima and Sher.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

