Understanding of Crucial Factors for Improving the Energy Density of Lithium-Sulfur Pouch Cells
- PMID: 35586266
- PMCID: PMC9108244
- DOI: 10.3389/fchem.2022.888750
Understanding of Crucial Factors for Improving the Energy Density of Lithium-Sulfur Pouch Cells
Abstract
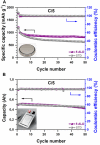
Rechargeable lithium-sulfur (Li-S) batteries are the most promising next-generation energy storage system owing to their high energy density and low cost. Despite the increasing number of publications on the Li-S technology, the number of studies on real prototype cells is rather low. Furthermore, novel concepts developed using small lab cells cannot simply be transferred to high-energy cell prototypes due to the fundamental differences. The electrolyte and lithium anode excess used in small lab cells is known to have a huge impact on the cycle life, capacity, and rate capability of the Li-S system. This work analyses the performance of pouch cell prototypes demonstrating the potential and hurdles of the technology. The impact of electrolyte variations and the sulfur cathode loading are studied. The energy density of Li-S pouch cell is improved up to 436 Wh kg-1 by a combination of different approaches related to cell manufacturing, sulfur cathode optimization, and electrolyte amount adjustment.
Keywords: cell balancing; electrolyte volume; high energy density; lithium-sulfur battery; pouch cell performance; sulfur loading density.
Copyright © 2022 Leonet, Doñoro, Fernández-Barquín, Kvasha, Urdampilleta and Blázquez.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Abraham K. M. (2012). Rechargeable Batteries for the 300-Mile Electric Vehicle and beyond. ECS Trans. 41, 27–34. 10.1149/1.3702853 - DOI
-
- Azaceta E., García S., Leonet O., Beltrán M., Gómez I., Chuvilin A., et al. (2020). Particle Atomic Layer Deposition as an Effective Way to Enhance Li-S Battery Energy Density. Mater. Today Energ. 18, 100567. 10.1016/j.mtener.2020.100567 - DOI
-
- Chabu J. M., Zeng K., Walle M. D., You X., Zhang M., Li Y., et al. (2017). Facile Fabrication of Sulfur/Graphene Composite for High-Rate Lithium-Sulfur Batteries. ChemistrySelect 2, 11035–11039. 10.1002/slct.201702336 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

