Isolation of five different primary cell types from a single sample of human skin
- PMID: 35586317
- PMCID: PMC9108981
- DOI: 10.1016/j.xpro.2022.101378
Isolation of five different primary cell types from a single sample of human skin
Abstract
We have developed a technique to isolate primary keratinocytes, melanocytes, fibroblasts, preadipocytes, and microvascular endothelial cells from an individual sample of human skin. The protocol describes step-by-step instructions for processing, cells isolation, and culture of neonatal foreskin, with adaptation for more demanding adult tissues. The availability of multiple isogenic cell types derived from individual skin samples offers the ability to investigate various areas of biology, in the context of cell-type specificity without potential confounding influence of inter-individual or genetic differences. For complete details on the use and execution of this protocol, please refer to Holliman et al. (2017), Horvath et al. (2019), Horvath et al. (2018), Kabacik et al. (2018), Lowe et al. (2020), Lu et al. (2019), and Lu et al. (2018).
Keywords: Cell Biology; Cell culture; Cell isolation; Clinical Protocol; Health Sciences.
© 2022 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures
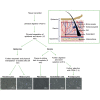



Similar articles
-
Isolation and feeder-free primary culture of four cell types from a single human skin sample.STAR Protoc. 2022 Feb 10;3(1):101172. doi: 10.1016/j.xpro.2022.101172. eCollection 2022 Mar 18. STAR Protoc. 2022. PMID: 35199036 Free PMC article.
-
An optimized protocol to identify keratinocyte subpopulations in vitro by single-cell RNA sequencing analysis.STAR Protoc. 2022 Dec 16;3(4):101906. doi: 10.1016/j.xpro.2022.101906. Epub 2022 Dec 6. STAR Protoc. 2022. PMID: 36595953 Free PMC article.
-
A Method for Isolating and Culturing Skin Cells: Application to Endothelial Cells, Fibroblasts, Keratinocytes, and Melanocytes From Punch Biopsies in Systemic Sclerosis Skin.Front Immunol. 2020 Oct 7;11:566607. doi: 10.3389/fimmu.2020.566607. eCollection 2020. Front Immunol. 2020. PMID: 33117350 Free PMC article.
-
Isolation, culture, and transfection of melanocytes.Curr Protoc Cell Biol. 2014 Jun 3;63:1.8.1-20. doi: 10.1002/0471143030.cb0108s63. Curr Protoc Cell Biol. 2014. PMID: 24894835 Review.
-
Autocrine and paracrine regulation of melanocytes in human skin and in pigmentary disorders.Pigment Cell Res. 2004 Apr;17(2):96-110. doi: 10.1111/j.1600-0749.2003.00126.x. Pigment Cell Res. 2004. PMID: 15016298 Review.
Cited by
-
Efficient protocol for isolating human fibroblast from primary skin cell cultures: application to keloid, hypertrophic scar, and normal skin biopsies.Biol Methods Protoc. 2024 Oct 29;9(1):bpae082. doi: 10.1093/biomethods/bpae082. eCollection 2024. Biol Methods Protoc. 2024. PMID: 39554256 Free PMC article.
-
Rebuilding the microenvironment of primary tumors in humans: a focus on stroma.Exp Mol Med. 2024 Mar;56(3):527-548. doi: 10.1038/s12276-024-01191-5. Epub 2024 Mar 5. Exp Mol Med. 2024. PMID: 38443595 Free PMC article. Review.
-
Efficient and simple protocol for mechanical isolation of foreskin-derived primary human dermal fibroblasts for replicative senescence studies.BMC Res Notes. 2025 Jul 18;18(1):311. doi: 10.1186/s13104-025-07373-2. BMC Res Notes. 2025. PMID: 40682140 Free PMC article.
References
-
- Dickson M.A., Hahn W.C., Ino Y., Ronfard V., Wu J.Y., Weinberg R.A., Louis D.N., Li F.P., Rheinwald J.G. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol. Cell Biol. 2000;20:1436–1447. doi: 10.1128/mcb.20.4.1436-1447.2000. - DOI - PMC - PubMed
-
- Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S., Bellamy D., Bibi S., Bittaye M., Clutterbuck E.A., et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

