Small Molecule Inhibitors of TET Dioxygenases: Bobcat339 Activity Is Mediated by Contaminating Copper(II)
- PMID: 35586434
- PMCID: PMC9109264
- DOI: 10.1021/acsmedchemlett.1c00677
Small Molecule Inhibitors of TET Dioxygenases: Bobcat339 Activity Is Mediated by Contaminating Copper(II)
Abstract
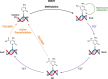
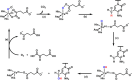
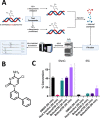
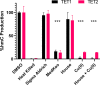
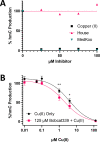
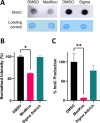
Ten eleven translocation (TET) dioxygenases 1-3 are non-heme Fe(II) and α-ketoglutarate dependent enzymes that catalyze oxidation of 5-methylcytosine (5mC) in DNA to hydroxymethyl-C, formyl-C, and carboxy-C. This typically leads to gene activation and epigenetic remodeling. Most known inhibitors of TET are α-ketoglutarate mimics that may interfere with other α-ketoglutarate dependent enzymes. Recently, a novel cytosine-based inhibitor of TET, Bobcat339, was reported to have mid-μM inhibitory activity against TET1 and TET2. The molecule is now sold as a TET inhibitor by several vendors. We independently prepared Bobcat339 in our laboratory and observed that it had minimal inhibitory activity against human TET1 and TET2 via a quantitative LC-ESI-MS/MS assay. Furthermore, the inhibitory activity of commercial Bobcat339 preparations was directly correlated with Cu(II) content. We therefore conclude that Bobcat339 alone is not capable of inhibiting TET enzymes at the reported concentrations, and that its activity is enhanced by contaminating Cu(II).
© 2022 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Mulholland C. B.; Traube F. R.; Ugur E.; Parsa E.; Eckl E. M.; Schonung M.; Modic M.; Bartoschek M. D.; Stolz P.; Ryan J.; Carell T.; Leonhardt H.; Bultmann S. Distinct and stage-specific contributions of TET1 and TET2 to stepwise cytosine oxidation in the transition from naive to primed pluripotency. Sci. Rep 2020, 10 (1), 12066. 10.1038/s41598-020-68600-3. - DOI - PMC - PubMed
-
- Seiler C. L.; Fernandez J.; Koerperich Z.; Andersen M. P.; Kotandeniya D.; Nguyen M. E.; Sham Y. Y.; Tretyakova N. Y. Maintenance DNA Methyltransferase Activity in the Presence of Oxidized Forms of 5-Methylcytosine: Structural Basis for Ten Eleven Translocation-Mediated DNA Demethylation. Biochemistry 2018, 57 (42), 6061–6069. 10.1021/acs.biochem.8b00683. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

