Is Pharmacogenetic Panel Testing Applicable to Low-Dose Methotrexate in Rheumatoid Arthritis? - A Case Report
- PMID: 35586477
- PMCID: PMC9109898
- DOI: 10.2147/PGPM.S354011
Is Pharmacogenetic Panel Testing Applicable to Low-Dose Methotrexate in Rheumatoid Arthritis? - A Case Report
Abstract
Purpose: Pharmacogenetic (PGx) panel testing could help to determine the heritable component of a rheumatoid arthritis (RA) patient's susceptibility for therapy failure and/or adverse drug reactions (ADRs) from methotrexate (MTX). Considering the literature mentioning the potential applicability of PGx panel testing within MTX regimens, we discuss the case of a patient who was treated with MTX, suffered from ADRs, and obtained a reactive PGx panel testing.
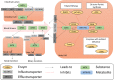
Genotyping: We used a commercial PGx panel test involving the ABC-transporters P-glycoprotein (P-gp; gene: ABCB1), and breast cancer resistance protein (BCRP; gene: ABCG2), the solute carriers reduced folate carrier 1 (RFC1; gene: SLC19A1), and organic anion transporting polypeptide 1B1 (OATP1B1; gene: SLCO1B1), and the enzymes inosine triphosphatase (ITPA), and glutathione transferase P1 (GSTP1). In addition, we genotyped the patient for the enzymes 5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase (AICAR)/inosine monophosphate (IMP) cyclohydrolase (gene name: ATIC), gamma-glutamyl hydrolase (gene name: GGH) and methylenetetrahydrofolate reductase (gene name: MTHFR).
Results: The PGx profile of the patient revealed genetic variants in SLC19A1, ABCB1, and MTHFR, which may explain the ADRs experienced during the treatment with MTX and a potentially lower efficacy of MTX. Based on our interpretation of the PGx profile, we recommended the patient to avoid MTX in the future.
Conclusion: The MTX pathway is complex, which makes the interpretation of genetic variants affecting metabolism challenging. A reactive PGx panel test was applicable to explain ADRs experienced during MTX treatment for a patient with RA. However, the clinical utility of PGx-guided MTX treatment in a primary care setting is still limited. In order to base a recommendation for MTX on PGx data, we need genome-wide association studies, large prospective multicenter studies and PGx studies, which analyze different multi-gene haplotypes and gene-drug-drug interactions for MTX.
Keywords: ABCB1; MTHFR; MTX; PGx; SLC19A1; methotrexate; pharmacogenetics; rheumatoid arthritis.
© 2022 Jeiziner et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
References
-
- Jenko B, Tomšič M, Jekić B, Milić V, Dolžan V, Praprotnik S. Clinical pharmacogenetic models of treatment response to methotrexate monotherapy in Slovenian and Serbian rheumatoid arthritis patients: differences in patient’s management may preclude generalization of the models. Front Pharmacol. 2018;9. doi: 10.3389/fphar.2018.00020 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous