Influence of T-Bar on Calcium Concentration Impacting Release Probability
- PMID: 35586479
- PMCID: PMC9108211
- DOI: 10.3389/fncom.2022.855746
Influence of T-Bar on Calcium Concentration Impacting Release Probability
Abstract
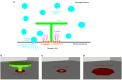
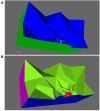
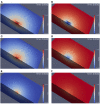
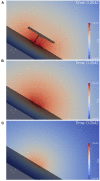
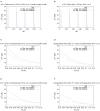
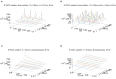
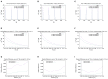
The relation of form and function, namely the impact of the synaptic anatomy on calcium dynamics in the presynaptic bouton, is a major challenge of present (computational) neuroscience at a cellular level. The Drosophila larval neuromuscular junction (NMJ) is a simple model system, which allows studying basic effects in a rather simple way. This synapse harbors several special structures. In particular, in opposite to standard vertebrate synapses, the presynaptic boutons are rather large, and they have several presynaptic zones. In these zones, different types of anatomical structures are present. Some of the zones bear a so-called T-bar, a particular anatomical structure. The geometric form of the T-bar resembles the shape of the letter "T" or a table with one leg. When an action potential arises, calcium influx is triggered. The probability of vesicle docking and neurotransmitter release is superlinearly proportional to the concentration of calcium close to the vesicular release site. It is tempting to assume that the T-bar causes some sort of calcium accumulation and hence triggers a higher release probability and thus enhances neurotransmitter exocytosis. In order to study this influence in a quantitative manner, we constructed a typical T-bar geometry and compared the calcium concentration close to the active zones (AZs). We compared the case of synapses with and without T-bars. Indeed, we found a substantial influence of the T-bar structure on the presynaptic calcium concentrations close to the AZs, indicating that this anatomical structure increases vesicle release probability. Therefore, our study reveals how the T-bar zone implies a strong relation between form and function. Our study answers the question of experimental studies (namely "Wichmann and Sigrist, Journal of neurogenetics 2010") concerning the sense of the anatomical structure of the T-bar.
Keywords: Drosophila larval NMJ; T-bar; UG4; VGCC; calcium influx; calcium microdomain; diffusion-reaction PDE model; vesicle release probability.
Copyright © 2022 Knodel, Dutta Roy and Wittum.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Impact of spatiotemporal calcium dynamics within presynaptic active zones on synaptic delay at the frog neuromuscular junction.J Neurophysiol. 2018 Feb 1;119(2):688-699. doi: 10.1152/jn.00510.2017. Epub 2017 Nov 22. J Neurophysiol. 2018. PMID: 29167324 Free PMC article.
-
Probabilistic secretion of quanta and the synaptosecretosome hypothesis: evoked release at active zones of varicosities, boutons, and endplates.Biophys J. 1997 Oct;73(4):1815-29. doi: 10.1016/S0006-3495(97)78212-2. Biophys J. 1997. PMID: 9336177 Free PMC article.
-
Synaptic structural complexity as a factor enhancing probability of calcium-mediated transmitter release.J Neurophysiol. 1996 Jun;75(6):2451-66. doi: 10.1152/jn.1996.75.6.2451. J Neurophysiol. 1996. PMID: 8793756
-
Presynaptic active zones in invertebrates and vertebrates.EMBO Rep. 2015 Aug;16(8):923-38. doi: 10.15252/embr.201540434. Epub 2015 Jul 9. EMBO Rep. 2015. PMID: 26160654 Free PMC article. Review.
-
The active zone T-bar--a plasticity module?J Neurogenet. 2010 Sep;24(3):133-45. doi: 10.3109/01677063.2010.489626. J Neurogenet. 2010. PMID: 20553221 Review.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

