"Prefrontal" Neuronal Foundations of Visual Asymmetries in Pigeons
- PMID: 35586719
- PMCID: PMC9108483
- DOI: 10.3389/fphys.2022.882597
"Prefrontal" Neuronal Foundations of Visual Asymmetries in Pigeons
Abstract
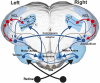
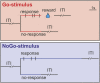
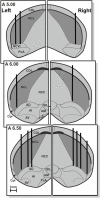
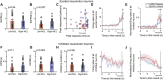
This study was conducted in order to reveal the possibly lateralized processes in the avian nidopallium caudolaterale (NCL), a functional analogue to the mammalian prefrontal cortex, during a color discrimination task. Pigeons are known to be visually lateralized with a superiority of the left hemisphere/right eye for visual feature discriminations. While animals were working on a color discrimination task, we recorded single visuomotor neurons in left and right NCL. As expected, pigeons learned faster and responded more quickly when seeing the stimuli with their right eyes. Our electrophysiological recordings discovered several neuronal properties of NCL neurons that possibly contributed to this behavioral asymmetry. We found that the speed of stimulus encoding was identical between left and right NCL but action generation was different. Here, most left hemispheric NCL neurons reached their peak activities shortly before response execution. In contrast, the majority of right hemispheric neurons lagged behind and came too late to control the response. Thus, the left NCL dominated the animals' behavior not by a higher efficacy of encoding, but by being faster in monopolizing the operant response. A further asymmetry concerned the hemisphere-specific integration of input from the contra- and ipsilateral eye. The left NCL was able to integrate and process visual input from the ipsilateral eye to a higher degree and thus achieved a more bilateral representation of two visual fields. We combine these novel findings with those from previous publications to come up with a working hypothesis that could explain how hemispheric asymmetries for visual feature discrimination in birds are realized by a sequential buildup of lateralized neuronal response properties in the avian forebrain.
Keywords: birds; lateralization; nidopallium caudolaterale; single unit recording; tectofugal system.
Copyright © 2022 Xiao and Güntürkün.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Asymmetrical Commissural Control of the Subdominant Hemisphere in Pigeons.Cell Rep. 2018 Oct 30;25(5):1171-1180.e3. doi: 10.1016/j.celrep.2018.10.011. Cell Rep. 2018. PMID: 30380409
-
Lateralized reward-related visual discrimination in the avian entopallium.Eur J Neurosci. 2012 Apr;35(8):1337-43. doi: 10.1111/j.1460-9568.2012.08049.x. Epub 2012 Mar 27. Eur J Neurosci. 2012. PMID: 22452655
-
The role of nidopallium caudolaterale in the goal-directed behavior of pigeons.Behav Brain Res. 2017 May 30;326:112-120. doi: 10.1016/j.bbr.2017.02.042. Epub 2017 Mar 10. Behav Brain Res. 2017. PMID: 28288807
-
Functional and structural comparison of visual lateralization in birds - similar but still different.Front Psychol. 2014 Mar 25;5:206. doi: 10.3389/fpsyg.2014.00206. eCollection 2014. Front Psychol. 2014. PMID: 24723898 Free PMC article. Review.
-
Visual lateralization in birds: from neurotrophins to cognition?Eur J Morphol. 1997 Oct;35(4):290-302. Eur J Morphol. 1997. PMID: 9290937 Review.
Cited by
-
Visual categories and concepts in the avian brain.Anim Cogn. 2023 Jan;26(1):153-173. doi: 10.1007/s10071-022-01711-8. Epub 2022 Nov 10. Anim Cogn. 2023. PMID: 36352174 Free PMC article. Review.
-
Dynamics and development of interhemispheric conflict solving in pigeons.Sci Rep. 2025 Jan 11;15(1):1655. doi: 10.1038/s41598-024-85058-9. Sci Rep. 2025. PMID: 39794396 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

