Bactericidal Effect of Cecropin A Fused Endolysin on Drug-Resistant Gram-Negative Pathogens
- PMID: 35586934
- PMCID: PMC9628910
- DOI: 10.4014/jmb.2205.05009
Bactericidal Effect of Cecropin A Fused Endolysin on Drug-Resistant Gram-Negative Pathogens
Abstract
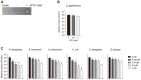
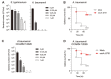
The rapid spread of superbugs leads to the escalation of infectious diseases, which threatens public health. Endolysins derived from bacteriophages are spotlighted as promising alternative antibiotics against multi-drug resistant bacteria. In this study, we isolated and characterized the novel Salmonella typhimurium phage PBST08. Bioinformatics analysis of the PBST08 genome revealed putative endolysin ST01 with a lysozyme-like domain. Since the lytic activity of the purified ST01 was minor, probably owing to the outer membrane, which blocks accessibility to peptidoglycan, antimicrobial peptide cecropin A (CecA) was fused to the N-terminus of ST01 to disrupt the outer membrane. The resulting CecA::ST01 has been shown to have increased bactericidal activity against gram-negative pathogens including Pseudomonas aeruginosa, Klebsiella pneumoniae, Acinetobacter baumannii, Escherichia coli, and Enterobacter cloacae and the most affected target was A. baumannii. In the presence of 0.25 μM CecA::ST01, A. baumannii ATCC 17978 strain was completely killed and CCARM 12026 strain was wiped out by 0.5 μM CecA::ST01, which is a clinical isolate of A. baumannii and resistant to multiple drugs including carbapenem. Moreover, the larvae of Galleria mellonella could be rescued up to 58% or 49% by the administration of CecA::ST01 upon infection by A. baumannii 17978 or CCARM 12026 strain. Finally, the antibacterial activity of CecA::ST01 was verified using 31 strains of five gram-negative pathogens by evaluation of minimal inhibitory concentration. Thus, the results indicate that a fusion of antimicrobial peptide to endolysin can enhance antibacterial activity and the spectrum of endolysin where multi-drug resistant gram-negative pathogens can be efficiently controlled.
Keywords: Endolysin; antimicrobial peptide; bacteriophage; gram-negative pathogens; multiple-drug resistance.
Conflict of interest statement
The authors have no financial conflicts of interest to declare.
Figures

Similar articles
-
In vitro and in vivo efficacy studies of an engineered endolysin targeting Gram-negative pathogens.Int J Biol Macromol. 2025 Apr;302:140463. doi: 10.1016/j.ijbiomac.2025.140463. Epub 2025 Jan 28. Int J Biol Macromol. 2025. PMID: 39884635
-
Characterization of Salmonella endolysin XFII produced by recombinant Escherichia coli and its application combined with chitosan in lysing Gram-negative bacteria.Microb Cell Fact. 2022 Aug 23;21(1):171. doi: 10.1186/s12934-022-01894-2. Microb Cell Fact. 2022. PMID: 35999567 Free PMC article.
-
A Novel Acinetobacter baumannii Bacteriophage Endolysin LysAB54 With High Antibacterial Activity Against Multiple Gram-Negative Microbes.Front Cell Infect Microbiol. 2021 Mar 2;11:637313. doi: 10.3389/fcimb.2021.637313. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33738267 Free PMC article.
-
Engineering strategies and challenges of endolysin as an antibacterial agent against Gram-negative bacteria.Microb Biotechnol. 2024 Apr;17(4):e14465. doi: 10.1111/1751-7915.14465. Microb Biotechnol. 2024. PMID: 38593316 Free PMC article. Review.
-
From endolysins to Artilysin®s: novel enzyme-based approaches to kill drug-resistant bacteria.Biochem Soc Trans. 2016 Feb;44(1):123-8. doi: 10.1042/BST20150192. Biochem Soc Trans. 2016. PMID: 26862197 Review.
Cited by
-
Retrospective analysis of the impact of pathogen spectrum and antibiotic resistance on the treatment efficacy of respiratory tract infections from 2012 to 2022.Am J Transl Res. 2025 Jan 15;17(1):480-488. doi: 10.62347/XZCT4326. eCollection 2025. Am J Transl Res. 2025. PMID: 39959241 Free PMC article.
-
The antibacterial activity of a novel highly thermostable endolysin, LysKP213, against Gram-negative pathogens is enhanced when combined with outer membrane permeabilizing agents.Front Microbiol. 2024 Oct 8;15:1454618. doi: 10.3389/fmicb.2024.1454618. eCollection 2024. Front Microbiol. 2024. PMID: 39439944 Free PMC article.
-
Development of sensitizer peptide-fused endolysin Lys1S-L9P acting against multidrug-resistant gram-negative bacteria.Front Microbiol. 2023 Nov 23;14:1296796. doi: 10.3389/fmicb.2023.1296796. eCollection 2023. Front Microbiol. 2023. PMID: 38075915 Free PMC article.
-
The Application and Limitations of Promising Biological Therapies in Livestock Production under the Context of Antibiotic Restrictions.Probiotics Antimicrob Proteins. 2025 Aug 14. doi: 10.1007/s12602-025-10705-0. Online ahead of print. Probiotics Antimicrob Proteins. 2025. PMID: 40810777 Review.
-
The Unique Capability of Endolysin to Tackle Antibiotic Resistance: Cracking the Barrier.J Xenobiot. 2025 Jan 25;15(1):19. doi: 10.3390/jox15010019. J Xenobiot. 2025. PMID: 39997362 Free PMC article. Review.
References
-
- Magiorakos A-P, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drugresistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

