Hepatocellular cystathionine γ lyase/hydrogen sulfide attenuates nonalcoholic fatty liver disease by activating farnesoid X receptor
- PMID: 35586979
- PMCID: PMC9795901
- DOI: 10.1002/hep.32577
Hepatocellular cystathionine γ lyase/hydrogen sulfide attenuates nonalcoholic fatty liver disease by activating farnesoid X receptor
Abstract
Background and aims: Hydrogen sulfide (H2 S) plays a protective role in NAFLD. However, whether cystathionine γ lyase (CSE), a dominant H2 S generating enzyme in hepatocytes, has a role in the pathogenesis of NAFLD is currently unclear.
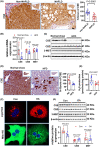
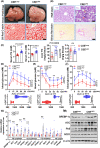
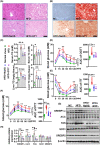
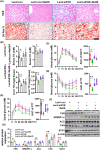
Approach and results: We showed that CSE protein expression is dramatically downregulated, especially in fibrotic areas, in livers from patients with NAFLD. In high-fat diet (HFD)-induced NAFLD mice or an oleic acid-induced hepatocyte model, the CSE/H2 S pathway is also downregulated. To illustrate a regulatory role for CSE in NAFLD, we generated a hepatocyte-specific CSE knockout mouse (CSELKO ). Feeding an HFD to CSELKO mice, they showed more hepatic lipid deposition with increased activity of the fatty acid de novo synthesis pathway, increased hepatic insulin resistance, and higher hepatic gluconeogenic ability compared to CSELoxp control mice. By contrast, H2 S donor treatment attenuated these phenotypes. Furthermore, the protection conferred by H2 S was blocked by farnesoid X receptor (FXR) knockdown. Consistently, serum deoxycholic acid and lithocholic acid (FXR antagonists) were increased, and tauro-β-muricholic acid (FXR activation elevated) was reduced in CSELKO . CSE/H2 S promoted a post-translation modification (sulfhydration) of FXR at Cys138/141 sites, thereby enhancing its activity to modulate expression of target genes related to lipid and glucose metabolism, inflammation, and fibrosis. Sulfhydration proteomics in patients' livers supported the CSE/H2 S modulation noted in the CSELKO mice.
Conclusions: FXR sulfhydration is a post-translational modification affected by hepatic endogenous CSE/H2 S that may promote FXR activity and attenuate NAFLD. Hepatic CSE deficiency promotes development of nonalcoholic steatohepatitis. The interaction between H2 S and FXR may be amenable to therapeutic drug treatment in NAFLD.
© 2022 The Authors. Hepatology published by Wiley Periodicals LLC on behalf of American Association for the Study of Liver Diseases.
Conflict of interest statement
Nothing to report.
Figures



References
-
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease‐meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. - PubMed
-
- Cruz‐Ramón V, Chinchilla‐López P, Ramírez‐Pérez O, Méndez‐Sánchez N. Bile acids in nonalcoholic fatty liver disease: new concepts and therapeutic advances. Ann Hepatol. 2017;16:s58–67. - PubMed
-
- Mudaliar S, Henry RR, Sanyal AJ, Morrow L, Marschall HU, Kipnes M, et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574–82.e1. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

