Myeloid CD40 deficiency reduces atherosclerosis by impairing macrophages' transition into a pro-inflammatory state
- PMID: 35587037
- PMCID: PMC10202633
- DOI: 10.1093/cvr/cvac084
Myeloid CD40 deficiency reduces atherosclerosis by impairing macrophages' transition into a pro-inflammatory state
Abstract
Aims: CD40 and its ligand, CD40L, play a critical role in driving atherosclerotic plaque development. Disrupted CD40-signalling reduces experimental atherosclerosis and induces a favourable stable plaque phenotype. We recently showed that small molecule-based inhibition of CD40-tumour necrosis factor receptor associated factor-6 interactions attenuates atherosclerosis in hyperlipidaemic mice via macrophage-driven mechanisms. The present study aims to detail the function of myeloid CD40 in atherosclerosis using myeloid-specific CD40-deficient mice.
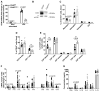
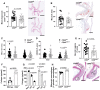
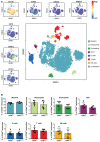
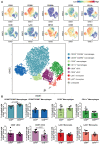
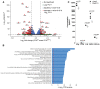
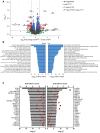
Method and results: Cd40flox/flox and LysM-cre Cd40flox/flox mice on an Apoe-/- background were generated (CD40wt and CD40mac-/-, respectively). Atherosclerotic lesion size, as well as plaque macrophage content, was reduced in CD40mac-/- compared to CD40wt mice, and their plaques displayed a reduction in necrotic core size. Transcriptomics analysis of the CD40mac-/- atherosclerotic aorta revealed downregulated pathways of immune pathways and inflammatory responses. Loss of CD40 in macrophages changed the representation of aortic macrophage subsets. Mass cytometry analysis revealed a higher content of a subset of alternative or resident-like CD206+CD209b- macrophages in the atherosclerotic aorta of CD40mac-/- compared to CD40wt mice. RNA-sequencing of bone marrow-derived macrophages of CD40mac-/- mice demonstrated upregulation of genes associated with alternatively activated macrophages (including Folr2, Thbs1, Sdc1, and Tns1).
Conclusions: We here show that absence of CD40 signalling in myeloid cells reduces atherosclerosis and limits systemic inflammation by preventing a shift in macrophage polarization towards pro-inflammatory states. Our study confirms the merit of macrophage-targeted inhibition of CD40 as a valuable therapeutic strategy to combat atherosclerosis.
Keywords: Atherosclerosis; CD40; Inflammation; Macrophage.
© The Author(s) 2022. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflicts of interest: C.W., D.A., E.L. and N.G. are supported by the Deutsche Forschungs Gemeinschaft (grants CRC1123, A5, SFB 1123, TRR259, SFB1116). E.L is also supported by the Netherlands CardioVascular Research Initiative, the Dutch Heart Foundation, Dutch Federation of University Medical Centers, the Netherlands Organization for Health Research and Development and the Royal Netherlands Academy of Sciences’ for the GENIUS project ‘Generating the best evidence-based pharmaceutical targets for atherosclerosis’ (CVON2011-19), and the ERC Con grant (CD40-INN). E.L. is also the vice chair at the ESC working group on atherosclerosis, serves on the EAS programme committee and the ATVB awards and programme committee and has received payment or honoraria for lectures at Novartis and Novo Nordisk. The remaining authors have nothing to disclose.
Figures


Similar articles
-
Deficiency of Endothelial CD40 Induces a Stable Plaque Phenotype and Limits Inflammatory Cell Recruitment to Atherosclerotic Lesions in Mice.Thromb Haemost. 2021 Nov;121(11):1530-1540. doi: 10.1055/a-1397-1858. Epub 2021 May 13. Thromb Haemost. 2021. PMID: 33618394
-
Silencing of CD40 in vivo reduces progression of experimental atherogenesis through an NF-κB/miR-125b axis and reveals new potential mediators in the pathogenesis of atherosclerosis.Atherosclerosis. 2016 Dec;255:80-89. doi: 10.1016/j.atherosclerosis.2016.11.002. Epub 2016 Nov 2. Atherosclerosis. 2016. PMID: 27835742
-
Myeloid cell-specific Irf5 deficiency stabilizes atherosclerotic plaques in Apoe-/- mice.Mol Metab. 2021 Nov;53:101250. doi: 10.1016/j.molmet.2021.101250. Epub 2021 May 12. Mol Metab. 2021. PMID: 33991749 Free PMC article.
-
CD40 signaling in vascular cells: a key role in atherosclerosis?Atherosclerosis. 1998 Apr;137 Suppl:S89-95. doi: 10.1016/s0021-9150(97)00309-2. Atherosclerosis. 1998. PMID: 9694547 Review.
-
Macrophage subsets in atherosclerosis as defined by single-cell technologies.J Pathol. 2020 Apr;250(5):705-714. doi: 10.1002/path.5392. Epub 2020 Mar 11. J Pathol. 2020. PMID: 32003464 Free PMC article. Review.
Cited by
-
Photodynamic Therapy for Atherosclerosis.Int J Mol Sci. 2024 Feb 6;25(4):1958. doi: 10.3390/ijms25041958. Int J Mol Sci. 2024. PMID: 38396639 Free PMC article. Review.
-
Vulnerable Atherosclerotic Plaque: Is There a Molecular Signature?Int J Mol Sci. 2022 Nov 7;23(21):13638. doi: 10.3390/ijms232113638. Int J Mol Sci. 2022. PMID: 36362423 Free PMC article. Review.
-
Macrophages Unmasked: Their Pivotal Role in Driving Atherosclerosis in Systemic Lupus Erythematosus.Clin Rev Allergy Immunol. 2025 Feb 7;68(1):10. doi: 10.1007/s12016-025-09025-6. Clin Rev Allergy Immunol. 2025. PMID: 39920534 Review.
-
Current status and challenges of multi-omics research using animal models of atherosclerosis.J Mol Cell Cardiol Plus. 2025 Jul 10;13:100476. doi: 10.1016/j.jmccpl.2025.100476. eCollection 2025 Sep. J Mol Cell Cardiol Plus. 2025. PMID: 40726539 Free PMC article. Review.
-
Bufei Jiedu Formula enhances CD40 activation and macrophage polarization to eliminate intracellular MRSA persisters.Front Immunol. 2025 Jul 17;16:1623182. doi: 10.3389/fimmu.2025.1623182. eCollection 2025. Front Immunol. 2025. PMID: 40746551 Free PMC article.
References
-
- Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, Tokgozoglu L, Lewis EF. Atherosclerosis. Nat Rev Dis Primers 2019;5:56. - PubMed
-
- Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, Mam V, Hasan A, Rosenberg Y, Iturriaga E, Gupta M, Tsigoulis M, Verma S, Clearfield M, Libby P, Goldhaber SZ, Seagle R, Ofori C, Saklayen M, Butman S, Singh N, Le May M, Bertrand O, Johnston J, Paynter NP, Glynn RJ, Investigators C. Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med 2019;380:752–762. - PMC - PubMed
-
- Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ, CANTOS Trial Group . Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. - PubMed
-
- Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, Berry C, Lopez-Sendon J, Ostadal P, Koenig W, Angoulvant D, Gregoire JC, Lavoie MA, Dube MP, Rhainds D, Provencher M, Blondeau L, Orfanos A, L’Allier PL, Guertin MC, Roubille F. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med 2019;381:2497–2505. - PubMed
-
- Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol 2013;61:404–410. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

