Birth of mice from meiotically arrested spermatocytes following biparental meiosis in halved oocytes
- PMID: 35587095
- PMCID: PMC9253789
- DOI: 10.15252/embr.202254992
Birth of mice from meiotically arrested spermatocytes following biparental meiosis in halved oocytes
Abstract
Microinjection of spermatozoa or spermatids into oocytes is a major choice for infertility treatment. However, the use of premeiotic spermatocytes has never been considered because of its technical problems. Here, we show that the efficiency of spermatocyte injection in mice can be improved greatly by reducing the size of the recipient oocytes. Live imaging showed that the underlying mechanism involves reduced premature separation of the spermatocyte's meiotic chromosomes, which produced much greater (19% vs. 1%) birth rates in smaller oocytes. Application of this technique to spermatocyte arrest caused by STX2 deficiency, an azoospermia factor also found in humans, resulted in the production of live offspring. Thus, the microinjection of primary spermatocytes into oocytes may be a potential treatment for overcoming a form of nonobstructive azoospermia caused by meiotic failure.
Keywords: azoospermia; fertilization; meiosis; oocyte; spermatocyte.
© 2022 The Authors.
Figures
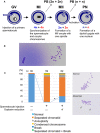
The scheme of construction of a diploid fertilized oocyte using a primary spermatocyte and a GV‐stage oocyte. The chromosomes of the spermatocyte and the oocyte are intermingled at MI to form a single chromosomal mass.
Spermatocyte chromosomes that underwent condensation (right) within a MII oocyte (MII chromosome mass, left). In this study, spermatocytes picked up for injection had tetrad chromosomes ready for condensation.
Chromosomal analysis of MII oocytes that had been injected with primary spermatocytes. In the spermatocyte‐injected groups, normality was improved by reducing the ooplasm mass (*P < 0.005 by Fisher’s exact probability test). Arrows in the right figure indicate prematurely separated chromatids. The numbers of oocytes observed are indicated on the top of the bars. For the exact numbers in each case, see also Appendix Table S1. PB, polar body; GV, germinal vesicle; MI, meiosis I; PN, pronuclear stage.
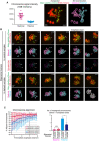
Identification of parental origin of the chromosomes was distinguishable based on H2B–mCherry fluorescent intensities (paternal chromosomes exhibit lower intensities; n = 20 oocytes). Bars and error bars denote means ± SD. The z‐projection image shows major satellite–mClover (centromeres, green) and H2B–mCherry (chromosomes, red). Time from anaphase onset is shown in h:min. Scale bar = 4 μm. The 3D‐reconstructed image shows maternal (magenta) and paternal (cyan) chromosomes. Spots indicate centromeres.
Chromosome tracking in 3D. The reconstructed images are viewed from the side of the metaphase plate. Signals are interpolated in the Z axis for visualization. White and red arrowheads, as well as red surfaces, indicate univalent‐like chromosomes that underwent unbalanced predivision (premature segregation of sister chromatids). Scale bar = 4 μm.
Halving the recipient ooplasmic mass rescued chromosome alignment. The numbers of misaligned chromosomes and their parental origin were determined in 3D (n = 39 and 17 oocytes). Error bars show the standard deviation. Student’s t‐test was used to compare means. *P < 0.05. N.S., not significant.
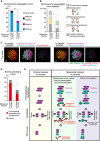
Halving the recipient ooplasm mass reduced chromosome segregation errors. Errors were determined by tracking all chromosomes at anaphase (n = 39 and 17 oocytes; See also Fig 2B). The parental origin of errors is shown. Ooplasmic halving significantly reduced the rate of errors (**P < 0.01, Chi‐square test).
Predivision was predominant in biparental meiosis. Chromosome segregation error patterns were categorized based on anaphase trajectories: nondisjunction (0:4 segregation), balanced predivision (2:2 sister chromatid segregation), unbalanced predivision (1:3 segregation including sister chromatid segregation), and complex patterns including predivision (multiple errors including sister chromatid segregation). Chromosome breakages (chromosomes lacking centromeres) were also observed. Data from 39 and 17 oocytes, respectively.
Univalent‐like chromosomes. Images were 3D‐reconstructed as in Fig 2B and viewed from the top of the metaphase plate. Red surfaces with white arrowheads indicate univalent‐like chromosomes. Scale bar = 3 μm.
Halving the ooplasm volume suppressed the premature separation of paternal chromosomes. Oocytes were categorized based on whether the chromosomes exhibited premature separation into univalent‐like structures prior to segregation errors. Data from 39 and 17 oocytes, respectively.
Summary of biparental meiosis. In normal‐sized oocytes, biparental meiosis frequently exhibits premature separation of paternal chromosomes into univalent‐like structures. These chromosomes undergo predivision (premature segregation of sister chromatids), and thus result in separated chromatids in MII oocytes. Chromosome nondisjunction and breakage were relatively minor. Halving the ooplasmic volume reduced premature separation of paternal chromosomes.
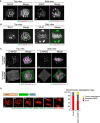
SGO2 localizes at the centromeres of spermatocyte‐derived and maternal chromosomes. Oocytes were fixed at metaphase I and stained for SGO2 (green), ACA (centromeres, magenta), and Hoechst33342 (DNA, blue). Images were reconstructed in 3D and viewed from the top and side of the metaphase plate. Note that signals are interpolated in z.
PLK1 localizes at the kinetochores of spermatocyte‐derived and maternal chromosomes. PLK1 (green) localization was investigated as in (A).
MAD2 kinetochore localization is defective on spermatocyte‐derived chromosomes. The localization of the closed (active) form of MAD2 (C‐MAD2, green) was investigated as in (A). Note that the kinetochore enrichment of MAD2 was less on the chromosomes in half of the metaphase plate, which likely corresponded to spermatocyte‐derived chromosomes, in control and halved oocytes.
Effect of forced SAC activation. Oocytes expressing MAD1‐mNeonGreen‐CENP‐C (green), together with major satellite–mClover (green) and H2B–mCherry (red), were imaged. Time after the start of imaging (h:mm) is shown. Oocytes were categorized based on anaphase figures (n = 24, 23 oocytes).
Mouse pups born following spermatocyte injection (left) and the birth rates following embryo transfer (right). The numbers in the graph indicate: (number of pups born)/(number of embryos transferred). For detailed results, see also Appendix Table S2.
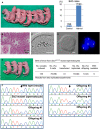
Histology of the testis of a Stx2repro34 mouse. Arrowheads indicate multinucleated cells containing spermatocyte‐like nuclei. There are no spermatids or spermatozoa. Bar = 50 μm.
An MII oocyte injected with a putative spermatocyte nucleus from a multinucleated cell in a Stx2repro34 mouse testis, showing the typical paired meiotic chromosomes. Bar = 20 μm.
A multinucleated cell isolated from a Stx2repro34 mouse testis, showing four nuclei. Differential interference contrast (left) and Hoechst‐stained (right) images. Bar = 10 μm.
Left: mouse pups born following microinjection with putative primary spermatocyte nuclei isolated from multinucleated cells; Right: birth rate of pups following Stx2repro34 spermatocyte microinjection.
Genomic sequencing confirming the origin of pups from Stx2repro34 spermatocytes. Arrows indicate the expected point mutation of Stx2repro34 . Y indicates a hybrid status with T and C bases.
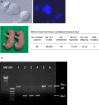
Multinucleated cells (syncytial spermatocytes) obtained from an Exoc1‐knockout male mouse. Each multinucleated cell contained 2–4 spermatocyte nuclei. Differential interference contrast microscopy (left) and Hoechst‐staining (right) images. Bar = 10 μm.
Mice born from Exoc1‐knockout spermatocytes.
Polymerase chain reaction analysis in mice born from Exoc1‐knockout spermatocytes. Mice #2, #3, and #4 carried the Exoc1‐knockout allele (del) while mice #1, #5, and #6 did not. Three pups that did not carry the Exoc1 mutation were most likely derived from spermatogonia that escaped the Cre‐induced Exoc1 deletion. The expected amplicon sizes are as follows: flox allele, 1,426 bp; wild type allele, 1,291 bp; deletion allele, 490 bp.


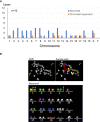
Chromosomal abnormalities were found in both autosomes and sex chromosomes.
A representative image of an oocyte with chromosomal aberrations. Arrows indicate prematurely separated chromatids. Besides them, chromosomes 5, 6, 14, 15 and 18 were numerically abnormal.
Similar articles
-
Fertilization and developmental initiation of oocytes by injection of spermatozoa and pre-spermatozoal cells.Ital J Anat Embryol. 2005;110(2 Suppl 1):145-50. Ital J Anat Embryol. 2005. PMID: 16101032 Review.
-
Mouse primary spermatocytes can complete two meiotic divisions within the oocyte cytoplasm.Biol Reprod. 1998 Jan;58(1):248-54. doi: 10.1095/biolreprod58.1.248. Biol Reprod. 1998. PMID: 9472948
-
Completion of meiosis in human primary spermatocytes through in vitro coculture with Vero cells.Fertil Steril. 2003 Mar;79 Suppl 1:795-801. doi: 10.1016/s0015-0282(02)04833-1. Fertil Steril. 2003. PMID: 12620493
-
Factors affecting meiotic and developmental competence of primary spermatocyte nuclei injected into mouse oocytes.Biol Reprod. 1998 Oct;59(4):871-7. doi: 10.1095/biolreprod59.4.871. Biol Reprod. 1998. PMID: 9746737
-
Fertilization of oocytes by injecting spermatozoa, spermatids and spermatocytes.Rev Reprod. 1996 Sep;1(3):149-52. doi: 10.1530/ror.0.0010149. Rev Reprod. 1996. PMID: 9414452 Review.
Cited by
-
Working in close quarters: biparental meiosis in the oocyte.EMBO Rep. 2022 Jul 5;23(7):e55360. doi: 10.15252/embr.202255360. Epub 2022 May 27. EMBO Rep. 2022. PMID: 35620872 Free PMC article.
-
The large cytoplasmic volume of oocyte.J Reprod Dev. 2023 Feb 8;69(1):1-9. doi: 10.1262/jrd.2022-101. Epub 2022 Nov 26. J Reprod Dev. 2023. PMID: 36436912 Free PMC article. Review.
References
-
- Akiyama K, Akimaru S, Asano Y, Khalaj M, Kiyosu C, Masoudi AA, Takahashi S, Katayama K, Tsuji T, Noguchi J et al (2008) A new ENU‐induced mutant mouse with defective spermatogenesis caused by a nonsense mutation of the Syntaxin 2/Epimorphin (Stx2/Epim) gene. J Reprod Dev 58: 122–128 - PubMed
-
- Chatot CL, Lewis JL, Torres I, Ziomek CA (1990) Development of 1‐cell embryos from different strains of mice in CZB medium. Biol Reprod 42: 432–440 - PubMed
-
- Cobb J, Cargile B, Handel MA (1999) Acquisition of competence to condense metaphase I chromosomes during spermatogenesis. Dev Biol 205: 49–64 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

