Integrin-linked kinase affects the sensitivity of esophageal squamous cell carcinoma cells to chemotherapy with cisplatin via the Wnt/beta-catenin signaling pathway
- PMID: 35587162
- PMCID: PMC9275978
- DOI: 10.1080/21655979.2022.2076497
Integrin-linked kinase affects the sensitivity of esophageal squamous cell carcinoma cells to chemotherapy with cisplatin via the Wnt/beta-catenin signaling pathway
Abstract
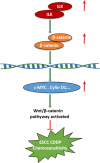
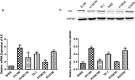
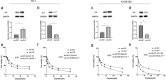
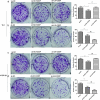
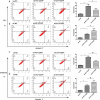
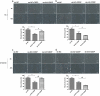
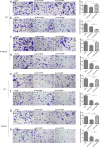
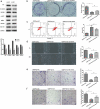
Recent studies have shown that the expression of integrin-linked kinase (ILK) was related to the occurrence, development, and malignant progression of esophageal squamous cell carcinoma (ESCC). However, research on the relationship between ILK and the chemosensitivity of ESCC has to date not been reported. The present study found that ILK was highly expressed in ESCC cell lines, and the overexpression of ILK in ESCC cells reduced the incidence of cell apoptosis and alleviated the cytotoxicity on cells induced by cisplatin (CDDP). Inversely, ILK knockdown increased CDDP-induced apoptosis and had an inhibitive effect on the malignant phenotype of ESCC, including cell proliferation, invasion, and migration. In addition, ILK knockdown in ESCC cells inhibited the expression of beta (β)-catenin and activated the wingless/integrated (Wnt) signaling pathway. Furthermore, cellular MYC (c-MYC) and Cylin D1 were the target genes of the Wnt signaling pathway. Rescue experiments showed that the overexpression of β-catenin reversed a tumor's inhibition and apoptosis abilities induced by ILK knockdown. In conclusion, ILK potentially reduced the CDDP sensitivity of ESCC cells by influencing the activity of the Wnt/β-catenin signaling pathway.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
References
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries [J]. CA Cancer J Clin. 2021:1–41. - PubMed
-
- Ohashi S, Miyamoto S, Kikuchi O, et al. Recent Advances From Basic and Clinical Studies of Esophageal Squamous Cell Carcinoma. Gastroenterology. 2015;149:1700–1715. - PubMed
-
- Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet. 2013;381:400–412. - PubMed
-
- Hanahan D, Weinberg RA.. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
