Clofoctol inhibits SARS-CoV-2 replication and reduces lung pathology in mice
- PMID: 35587469
- PMCID: PMC9119441
- DOI: 10.1371/journal.ppat.1010498
Clofoctol inhibits SARS-CoV-2 replication and reduces lung pathology in mice
Abstract
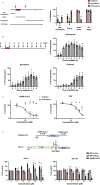
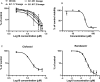
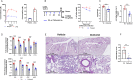
Drug repurposing has the advantage of shortening regulatory preclinical development steps. Here, we screened a library of drug compounds, already registered in one or several geographical areas, to identify those exhibiting antiviral activity against SARS-CoV-2 with relevant potency. Of the 1,942 compounds tested, 21 exhibited a substantial antiviral activity in Vero-81 cells. Among them, clofoctol, an antibacterial drug used for the treatment of bacterial respiratory tract infections, was further investigated due to its favorable safety profile and pharmacokinetic properties. Notably, the peak concentration of clofoctol that can be achieved in human lungs is more than 20 times higher than its IC50 measured against SARS-CoV-2 in human pulmonary cells. This compound inhibits SARS-CoV-2 at a post-entry step. Lastly, therapeutic treatment of human ACE2 receptor transgenic mice decreased viral load, reduced inflammatory gene expression and lowered pulmonary pathology. Altogether, these data strongly support clofoctol as a therapeutic candidate for the treatment of COVID-19 patients.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests:European Patent Application Serial No. EP20305633.8, entitled “Compound and method for the treatment of coronaviruses” related to this work was filed on 10 June 2020. Authors TB, LB, CM, SB, PB, ND, BD, JeD, EH, AM, YR and TV of this manuscript are inventors of the patent.
Figures


Similar articles
-
Suramin Inhibits SARS-CoV-2 Infection in Cell Culture by Interfering with Early Steps of the Replication Cycle.Antimicrob Agents Chemother. 2020 Jul 22;64(8):e00900-20. doi: 10.1128/AAC.00900-20. Print 2020 Jul 22. Antimicrob Agents Chemother. 2020. PMID: 32513797 Free PMC article.
-
Antiviral Activity of Type I, II, and III Interferons Counterbalances ACE2 Inducibility and Restricts SARS-CoV-2.mBio. 2020 Sep 10;11(5):e01928-20. doi: 10.1128/mBio.01928-20. mBio. 2020. PMID: 32913009 Free PMC article.
-
Cysteamine with In Vitro Antiviral Activity and Immunomodulatory Effects Has the Potential to Be a Repurposing Drug Candidate for COVID-19 Therapy.Cells. 2021 Dec 24;11(1):52. doi: 10.3390/cells11010052. Cells. 2021. PMID: 35011614 Free PMC article.
-
Inhibitors of endosomal acidification suppress SARS-CoV-2 replication and relieve viral pneumonia in hACE2 transgenic mice.Virol J. 2021 Feb 27;18(1):46. doi: 10.1186/s12985-021-01515-1. Virol J. 2021. PMID: 33639976 Free PMC article.
-
A new horizon for the old antibacterial drug clofoctol.Drug Discov Today. 2021 May;26(5):1302-1310. doi: 10.1016/j.drudis.2021.02.004. Epub 2021 Feb 10. Drug Discov Today. 2021. PMID: 33581321 Review.
Cited by
-
A community effort in SARS-CoV-2 drug discovery.Mol Inform. 2024 Jan;43(1):e202300262. doi: 10.1002/minf.202300262. Epub 2023 Nov 14. Mol Inform. 2024. PMID: 37833243 Free PMC article.
-
Discovery of Anti-Coronavirus Cinnamoyl Triterpenoids Isolated from Hippophae rhamnoides during a Screening of Halophytes from the North Sea and Channel Coasts in Northern France.Int J Mol Sci. 2023 Nov 22;24(23):16617. doi: 10.3390/ijms242316617. Int J Mol Sci. 2023. PMID: 38068938 Free PMC article.
-
Hyperforin, the major metabolite of St. John's wort, exhibits pan-coronavirus antiviral activity.Front Microbiol. 2024 Aug 8;15:1443183. doi: 10.3389/fmicb.2024.1443183. eCollection 2024. Front Microbiol. 2024. PMID: 39176276 Free PMC article.
-
A reporter cell line for the automated quantification of SARS-CoV-2 infection in living cells.Front Microbiol. 2022 Sep 29;13:1031204. doi: 10.3389/fmicb.2022.1031204. eCollection 2022. Front Microbiol. 2022. PMID: 36246297 Free PMC article.
-
Lichen or Associated Micro-Organism Compounds Are Active against Human Coronaviruses.Viruses. 2023 Aug 31;15(9):1859. doi: 10.3390/v15091859. Viruses. 2023. PMID: 37766264 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

