Systemic Immune Dysfunction in Cancer Patients Driven by IL6 Induction of LAG3 in Peripheral CD8+ T Cells
- PMID: 35587532
- PMCID: PMC10286228
- DOI: 10.1158/2326-6066.CIR-20-0736
Systemic Immune Dysfunction in Cancer Patients Driven by IL6 Induction of LAG3 in Peripheral CD8+ T Cells
Abstract
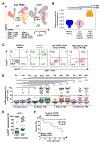
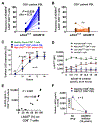
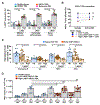
Many cancer patients do not develop a durable response to the current standard-of-care immunotherapies, despite substantial advances in targeting immune inhibitory receptors. A potential compounding issue, which may serve as an unappreciated, dominant resistance mechanism, is an inherent systemic immune dysfunction that is often associated with advanced cancer. Minimal response to inhibitory receptor (IR) blockade therapy and increased disease burden have been associated with peripheral CD8+ T-cell dysfunction, characterized by suboptimal T-cell proliferation and chronic expression of IRs (e.g., PD1 and LAG3). Here, we demonstrated that approximately a third of cancer patients analyzed in this study have peripheral CD8+ T cells that expressed robust intracellular LAG3 (LAG3IC), but not surface LAG3 (LAG3SUR) due to a disintegrin and metalloproteinase domain-containing protein 10 (ADAM10) cleavage. This is associated with poor disease prognosis and decreased CD8+ T-cell function, which could be partially reversed by anti-LAG3. Systemic immune dysfunction was restricted to CD8+ T cells, including, in some cases, a high percentage of peripheral naïve CD8+ T cells, and was driven by the cytokine IL6 via STAT3. These data suggest that additional studies are warranted to determine if the combination of increased LAG3IC in peripheral CD8+ T cells and elevated systemic IL6 can serve as predictive biomarkers and identify which cancer patients may benefit from LAG3 blockade.
©2022 American Association for Cancer Research.
Figures


Similar articles
-
Antibodies Against Immune Checkpoint Molecules Restore Functions of Tumor-Infiltrating T Cells in Hepatocellular Carcinomas.Gastroenterology. 2017 Oct;153(4):1107-1119.e10. doi: 10.1053/j.gastro.2017.06.017. Epub 2017 Jun 23. Gastroenterology. 2017. PMID: 28648905
-
Association Between Expression Level of PD1 by Tumor-Infiltrating CD8+ T Cells and Features of Hepatocellular Carcinoma.Gastroenterology. 2018 Dec;155(6):1936-1950.e17. doi: 10.1053/j.gastro.2018.08.030. Epub 2018 Aug 24. Gastroenterology. 2018. PMID: 30145359
-
Resistance to PD1 blockade in the absence of metalloprotease-mediated LAG3 shedding.Sci Immunol. 2020 Jul 17;5(49):eabc2728. doi: 10.1126/sciimmunol.abc2728. Sci Immunol. 2020. PMID: 32680952 Free PMC article.
-
Lymphocyte-activation gene 3 (LAG3): The next immune checkpoint receptor.Semin Immunol. 2019 Apr;42:101305. doi: 10.1016/j.smim.2019.101305. Semin Immunol. 2019. PMID: 31604537 Free PMC article. Review.
-
LAG3 (CD223) as a cancer immunotherapy target.Immunol Rev. 2017 Mar;276(1):80-96. doi: 10.1111/imr.12519. Immunol Rev. 2017. PMID: 28258692 Free PMC article. Review.
Cited by
-
T-cell and Soluble Co-inhibitory Receptor Expression in Patients With Visceral Leishmaniasis Are Markers of Treatment Response and Clinical Outcome.Open Forum Infect Dis. 2024 Oct 30;11(11):ofae649. doi: 10.1093/ofid/ofae649. eCollection 2024 Nov. Open Forum Infect Dis. 2024. PMID: 39564149 Free PMC article.
-
Regulatory T Cell Insufficiency in Autoimmune Diabetes Is Driven by Selective Loss of Neuropilin-1 on Intraislet Regulatory T Cells.J Immunol. 2024 Sep 15;213(6):779-794. doi: 10.4049/jimmunol.2300216. J Immunol. 2024. PMID: 39109924
-
A combined analysis of multi-omics data reveals the prognostic values and immunotherapy response of LAG3 in human cancers.Eur J Med Res. 2023 Dec 19;28(1):604. doi: 10.1186/s40001-023-01583-9. Eur J Med Res. 2023. PMID: 38115039 Free PMC article.
-
Oligoclonal CD4+CXCR5+ T cells with a cytotoxic phenotype appear in tonsils and blood.Commun Biol. 2024 Jul 18;7(1):879. doi: 10.1038/s42003-024-06563-1. Commun Biol. 2024. PMID: 39025930 Free PMC article.
-
Rheumatoid arthritis associated cytokines and therapeutics modulate immune checkpoint receptor expression on T cells.Front Immunol. 2025 Feb 6;16:1534462. doi: 10.3389/fimmu.2025.1534462. eCollection 2025. Front Immunol. 2025. PMID: 39981237 Free PMC article.
References
-
- Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gumus M, Mazieres J, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 2018;379:2040–51. - PubMed
-
- Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018;378:2078–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

