Pharmacokinetics of vaginal versus buccal misoprostol for labor induction at term
- PMID: 35587540
- PMCID: PMC9372425
- DOI: 10.1111/cts.13306
Pharmacokinetics of vaginal versus buccal misoprostol for labor induction at term
Abstract
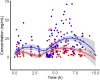
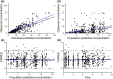
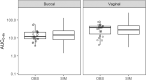
The IMPROVE study (NCT02408315) compared the efficacy and safety of vaginal and buccal administration of misoprostol for full-term, uncomplicated labor induction. This report compares the pharmacokinetics of misoprostol between vaginal and buccal routes. Women greater than or equal to 14 years of age undergoing induction of labor greater than or equal to 37 weeks gestation without significant complications were randomized to vaginal or buccal misoprostol 25 μg followed by 50 μg doses every 4 h. Misoprostol acid concentrations were determined using liquid chromatography-tandem mass spectrometry for the first 8 h in a subgroup of participants. A population pharmacokinetic model was developed using NONMEM. Plasma concentrations (n = 469) from 47 women were fit to a one-compartment nonlinear clearance model. The absorption rate constant (ka ) was dependent on both route and dose of administration: buccal 25 μg 0.724 (95% confidence interval, 0.54-0.92) h-1 ; 50 μg 0.531 (0.37-0.63) h-1 ; vaginal 25 μg 0.507 (0. 2-1. 4) h-1 ; and 50 μg 0.246 (0.103-0.453) h-1 . Relative bioavailability for vaginal compared to buccal route was 2.4 (1.63-4.77). There was no effect of body mass index or age on apparent clearance 705 (431-1099) L/h or apparent volume of distribution 632 (343-1008) L. The area under the concentration-time curve to 4 h following the first 25 μg dose of misoprostol was 16.5 (15.4-17.5) pg h/ml for buccal and 34.3 (32.5-36.1) pg h/ml for vaginal administration. The rate of buccal absorption was two times faster than that of vaginal, whereas bioavailability of vaginal administration was 2.4 times higher than that of buccal. Decreased time to delivery observed with vaginal dosing may be due to higher exposure to misoprostol acid compared to buccal.
© 2022 The Authors. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declare no competing interests for this work.
Figures
References
-
- Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Drake P. Births: final data for 2016. Natl Vital Stat Rep. 2018;67:1‐55. - PubMed
-
- ACOG . Practice bulletin no. 107: induction of labor. Obstet Gynecol. 2009;114:386‐397. - PubMed
-
- World Health Organization & Department of Reproductive Health and Research . WHO Recommendations for Induction of Labour; 2011. Accessed January 20, 2022. https://apps.who.int/iris/bitstream/handle/10665/44531/9789241501156_eng...
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

