Brucella activates the host RIDD pathway to subvert BLOS1-directed immune defense
- PMID: 35587649
- PMCID: PMC9119680
- DOI: 10.7554/eLife.73625
Brucella activates the host RIDD pathway to subvert BLOS1-directed immune defense
Abstract
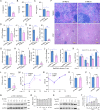
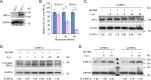
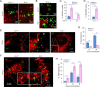
The phagocytosis and destruction of pathogens in lysosomes constitute central elements of innate immune defense. Here, we show that Brucella, the causative agent of brucellosis, the most prevalent bacterial zoonosis globally, subverts this immune defense pathway by activating regulated IRE1α-dependent decay (RIDD) of Bloc1s1 mRNA encoding BLOS1, a protein that promotes endosome-lysosome fusion. RIDD-deficient cells and mice harboring a RIDD-incompetent variant of IRE1α were resistant to infection. Inactivation of the Bloc1s1 gene impaired the ability to assemble BLOC-1-related complex (BORC), resulting in differential recruitment of BORC-related lysosome trafficking components, perinuclear trafficking of Brucella-containing vacuoles (BCVs), and enhanced susceptibility to infection. The RIDD-resistant Bloc1s1 variant maintains the integrity of BORC and a higher-level association of BORC-related components that promote centrifugal lysosome trafficking, resulting in enhanced BCV peripheral trafficking and lysosomal destruction, and resistance to infection. These findings demonstrate that host RIDD activity on BLOS1 regulates Brucella intracellular parasitism by disrupting BORC-directed lysosomal trafficking. Notably, coronavirus murine hepatitis virus also subverted the RIDD-BLOS1 axis to promote intracellular replication. Our work establishes BLOS1 as a novel immune defense factor whose activity is hijacked by diverse pathogens.
Keywords: BLOS1; Brucella; Brucella-containing vacuoles/lysosomes (BCVs); coronavirus; infectious disease; intracellular parasitism; microbiology; mouse; regulated IRE1α-dependent decay (RIDD).
© 2022, Wells et al.
Conflict of interest statement
KW, KH, AP, AC, DZ, JY, GG, YL, HC, XL, HZ, XF, Ld, RM, CJ, CM, JS, LB, KP, JL, AF, SS, JS, XQ, QQ, TF, Pd No competing interests declared
Figures









References
-
- Bright MD, Itzhak DN, Wardell CP, Morgan GJ, Davies FE. Cleavage of BLOC1S1 mRNA by IRE1 Is Sequence Specific, Temporally Separate from XBP1 Splicing, and Dispensable for Cell Viability under Acute Endoplasmic Reticulum Stress. Molecular and Cellular Biology. 2015;35:2186–2202. doi: 10.1128/MCB.00013-15. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

