Canonical Wnt signaling and the regulation of divergent mesenchymal Fgf8 expression in axolotl limb development and regeneration
- PMID: 35587651
- PMCID: PMC9154742
- DOI: 10.7554/eLife.79762
Canonical Wnt signaling and the regulation of divergent mesenchymal Fgf8 expression in axolotl limb development and regeneration
Abstract
The expression of fibroblast growth factors (Fgf) ligands in a specialized epithelial compartment, the Apical Ectodermal Ridge (AER), is a conserved feature of limb development across vertebrate species. In vertebrates, Fgf 4, 8, 9, and 17 are all expressed in the AER. An exception to this paradigm is the salamander (axolotl) developing and regenerating limb, where key Fgf ligands are expressed in the mesenchyme. The mesenchymal expression of Amex.Fgf8 in axolotl has been suggested to be critical for regeneration. To date, there is little knowledge regarding what controls Amex.Fgf8 expression in the axolotl limb mesenchyme. A large body of mouse and chick studies have defined a set of transcription factors and canonical Wnt signaling as the main regulators of epidermal Fgf8 expression in these organisms. In this study, we address the hypothesis that alterations to one or more of these components during evolution has resulted in mesenchymal Amex.Fgf8 expression in the axolotl. To sensitively quantify gene expression with spatial precision, we combined optical clearing of whole-mount axolotl limb tissue with single molecule fluorescent in situ hybridization and a semiautomated quantification pipeline. Several candidate upstream components were found expressed in the axolotl ectoderm, indicating that they are not direct regulators of Amex.Fgf8 expression. We found that Amex.Wnt3a is expressed in axolotl limb epidermis, similar to chicken and mouse. However, unlike in amniotes, Wnt target genes are activated preferentially in limb mesenchyme rather than in epidermis. Inhibition and activation of Wnt signaling results in downregulation and upregulation of mesenchymal Amex.Fgf8 expression, respectively. These results implicate a shift in tissue responsiveness to canonical Wnt signaling from epidermis to mesenchyme as one step contributing to the unique mesenchymal Amex.Fgf8 expression seen in the axolotl.
Keywords: Fgf8; HCR; Wnt; axolotl; developmental biology; limb regeneration; regenerative medicine; stem cells.
© 2022, Glotzer, Tardivo et al.
Conflict of interest statement
GG, PT, ET No competing interests declared
Figures





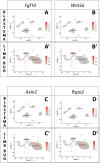


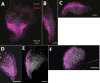

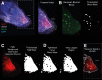


Similar articles
-
Fgf-signaling is compartmentalized within the mesenchyme and controls proliferation during salamander limb development.Elife. 2019 Sep 20;8:e48507. doi: 10.7554/eLife.48507. Elife. 2019. PMID: 31538936 Free PMC article.
-
Expression of fibroblast growth factors 4, 8, and 10 in limbs, flanks, and blastemas of Ambystoma.Dev Dyn. 2002 Mar;223(2):193-203. doi: 10.1002/dvdy.10049. Dev Dyn. 2002. PMID: 11836784
-
Expression patterns of Fgf-8 during development and limb regeneration of the axolotl.Dev Dyn. 2001 Jan;220(1):40-8. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1085>3.0.CO;2-8. Dev Dyn. 2001. PMID: 11146506
-
Mechanisms of retinoic acid signalling and its roles in organ and limb development.Nat Rev Mol Cell Biol. 2015 Feb;16(2):110-23. doi: 10.1038/nrm3932. Epub 2015 Jan 5. Nat Rev Mol Cell Biol. 2015. PMID: 25560970 Free PMC article. Review.
-
Putative epithelial-mesenchymal transitions during salamander limb regeneration: Current perspectives and future investigations.Ann N Y Acad Sci. 2024 Oct;1540(1):89-103. doi: 10.1111/nyas.15210. Epub 2024 Sep 13. Ann N Y Acad Sci. 2024. PMID: 39269330 Review.
Cited by
-
The evolution of cephalic fins in manta rays and their relatives: functional evidence for initiation of domain splitting and modulation of the Wnt signaling pathway in the pectoral fin AER of the little skate.Evodevo. 2024 Dec 27;15(1):17. doi: 10.1186/s13227-024-00233-3. Evodevo. 2024. PMID: 39731200 Free PMC article.
-
Multi-species atlas resolves an axolotl limb development and regeneration paradox.Nat Commun. 2023 Oct 10;14(1):6346. doi: 10.1038/s41467-023-41944-w. Nat Commun. 2023. PMID: 37816738 Free PMC article.
-
Sall4 regulates downstream patterning genes during limb regeneration.Dev Biol. 2024 Nov;515:151-159. doi: 10.1016/j.ydbio.2024.07.015. Epub 2024 Jul 25. Dev Biol. 2024. PMID: 39067503
-
Molecular basis of positional memory in limb regeneration.Nature. 2025 Jun;642(8068):730-738. doi: 10.1038/s41586-025-09036-5. Epub 2025 May 21. Nature. 2025. PMID: 40399677 Free PMC article.
-
Co-evolving infectivity and expression patterns drive the diversification of endogenous retroviruses.EMBO J. 2025 Jun 5. doi: 10.1038/s44318-025-00471-8. Online ahead of print. EMBO J. 2025. PMID: 40474005
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- SRA/SRX7140465
LinkOut - more resources
Full Text Sources
Other Literature Sources

