Characterization of critically ill patients with septic shock and sepsis-associated cardiomyopathy using cardiovascular MRI
- PMID: 35587684
- PMCID: PMC9288744
- DOI: 10.1002/ehf2.13938
Characterization of critically ill patients with septic shock and sepsis-associated cardiomyopathy using cardiovascular MRI
Abstract
Aims: Sepsis-induced cardiomyopathy is a major complication of septic shock and contributes to its high mortality. This pilot study investigated myocardial tissue differentiation in critically ill, sedated, and ventilated patients with septic shock using cardiovascular magnetic resonance (MR).
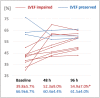
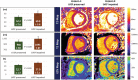
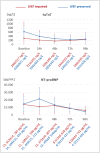
Methods and results: Fifteen patients with septic shock were prospectively recruited from the intensive care unit. Individuals received a cardiac MR scan (1.5 T) within 48 h after initial catecholamine peak and a transthoracic echocardiography at 48 and 96 h after cardiac MR. Left ventricular ejection fraction was assessed using both imaging modalities. During cardiac MR imaging, balanced steady-state free precession imaging was performed for evaluation of cardiac anatomy and function in long-axis and short-axis views. Native T1 maps (modified Look-Locker inversion recovery 5 s(3 s)3 s), T2 maps, and extracellular volume maps were acquired in mid-ventricular short axis and assessed for average plane values. Patients were given 0.2 mmol/kg of gadoteridol for extracellular volume quantification and late gadolinium enhancement imaging. Critical care physicians monitored sedated and ventilated patients during the scan with continuous invasive monitoring and realized breathholds through manual ventilation breaks. Laboratory analysis included high-sensitive troponine T and N terminal pro brain natriuretic peptide levels. Twelve individuals with complete datasets were available for analysis (age 59.5 ± 16.9 years; 6 female). Nine patients had impaired systolic function with left ventricular ejection fraction (LVEF) < 50% (39.8 ± 5.7%), and three individuals had preserved LVEF (66.9 ± 6.7%). Global longitudinal strain was impaired in both subgroups (LVEF impaired: 11.0 ± 1.8%; LVEF preserved: 16.0 ± 5.8%; P = 0.1). All patients with initially preserved LVEF died during hospital stay; in-hospital mortality with initially impaired LVEF was 11%. Upon echocardiographic follow-up, LVEF improved in all previously impaired patients at 48 (52.3 ± 9.0%, P = 0.06) and 96 h (54.9 ± 7.0%, P = 0.02). Patients with impaired systolic function had increased T2 times as compared with patients with preserved LVEF (60.8 ± 5.6 ms vs. 52.2 ± 2.8 ms; P = 0.02). Left ventricular GLS was decreased in all study individuals with impaired LVEF (11.0 ± 1.8%) and less impaired with preserved LVEF (16.0 ± 5.8%; P = 0.01). T1 mapping showed increased T1 times in patients with LVEF impairment as compared with patients with preserved LVEF (1093.9 ± 86.6 ms vs. 987.7 ± 69.3 ms; P = 0.03). Extracellular volume values were elevated in patients with LVEF impairment (27.9 ± 2.1%) as compared with patients with preserved LVEF (22.7 ± 1.9%; P < 0.01).
Conclusions: Septic cardiomyopathy with impaired LVEF reflects inflammatory cardiomyopathy. Takotsubo-like contractility patterns occur in some cases. Cardiac MR is safely feasible in critically ill, sedated, and ventilated patients using extensive monitoring and experienced staff.
Trial registration: retrospectively registered (ISRCTN85297773).
Keywords: CMR; Cardiac MR; Inflammation; Non-ischaemic cardiomyopathy; Sepsis; Septic cardiomyopathy; Septic shock.
© 2022 The Authors. ESC Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Conflict of interest statement
None declared.
Figures

References
-
- Parrillo JE, Parker MM, Natanson C, Suffredini AF, Danner RL, Cunnion RE, Ognibene FP. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med. 1990; 113: 227–242. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2197912 - PubMed
-
- Vieillard‐Baron A, Caille V, Charron C, Belliard G, Page B, Jardin F. Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit Care Med. 2008; 36: 1701–1706. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18496368 - PubMed
-
- Poelaert J, Declerck C, Vogelaers D, Colardyn F, Visser CA. Left ventricular systolic and diastolic function in septic shock. Intensive Care Med. 1997; 23: 553–560. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9201528 - PubMed
-
- Ehrman RR, Sullivan AN, Favot MJ, Sherwin RL, Reynolds CA, Abidov A, Levy PD. Pathophysiology, echocardiographic evaluation, biomarker findings, and prognostic implications of septic cardiomyopathy: a review of the literature. Crit Care. 2018; 22: 112. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29724231 - PMC - PubMed
-
- Madorin WS, Rui T, Sugimoto N, Handa O, Cepinskas G, Kvietys PR. Cardiac myocytes activated by septic plasma promote neutrophil transendothelial migration: role of platelet‐activating factor and the chemokines LIX and KC. Circ Res. 2004; 94: 944–951. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14988231 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

