Effect of Denosumab Added to 2 Different nab-Paclitaxel Regimens as Neoadjuvant Therapy in Patients With Primary Breast Cancer: The GeparX 2 × 2 Randomized Clinical Trial
- PMID: 35588050
- PMCID: PMC9121303
- DOI: 10.1001/jamaoncol.2022.1059
Effect of Denosumab Added to 2 Different nab-Paclitaxel Regimens as Neoadjuvant Therapy in Patients With Primary Breast Cancer: The GeparX 2 × 2 Randomized Clinical Trial
Abstract
Importance: Adjuvant denosumab might improve disease-free survival in hormone receptor (HR)-positive primary breast cancer (BC). The optimal neoadjuvant nab-paclitaxel schedule in terms of efficacy and safety is unclear.
Objective: To determine whether adding denosumab to anthracycline/taxane-containing neoadjuvant chemotherapy (NACT) increases the pathological complete response (pCR) rate and which nab-paclitaxel schedule is more effective in the NACT setting.
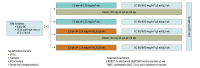
Design, setting, and participants: The GeparX was a multicenter, prospective, open-label, phase 2b, 2 × 2 randomized clinical trial conducted by GBG and AGO-B at 38 German sites between February 2017 and March 2019. The analysis data set was locked September 4, 2020; analysis was completed November 13, 2020. Patients had unilateral or bilateral primary BC, stage cT2-cT4a-d or cT1c, with either clinically node-positive or pathologically node-positive or HR-negative disease, or Ki-67 proliferation index greater than 20%, or ERBB2 (formerly HER2)-positive BC.
Interventions: Patients were randomized to receive or not receive denosumab, 120 mg subcutaneously every 4 weeks for 6 cycles, and either nab-paclitaxel, 125 mg/m2 weekly for 12 weeks or days 1 and 8 every 3 weeks for 4 cycles (8 doses), followed by 4 cycles of epirubicin/cyclophosphamide, 90/600 mg/m2 (every 2 weeks or every 3 weeks). Carboplatin was given in triple-negative BC (TNBC), and trastuzumab biosimilar ABP980 plus pertuzumab was given in ERBB2-positive BC (ERBB2-positive substudy).
Main outcomes and measures: The primary outcome was pCR rates between arms for each randomization.
Results: A total of 780 female (n = 779) and male (n = 1) patients (median [range] age, 49.0 [22-80] years) were randomized to the 4 treatment groups. The pCR (ypT0 ypN0) rate was 41.0% (90% CI, 37%-45%) with denosumab vs 42.8% (90% CI, 39%-47%) (P = .58) without denosumab, irrespective of BC subtype. Nab-paclitaxel weekly resulted in a significantly (significance level of α = .10) higher pCR rate of 44.9% (90% CI, 41%-49%) vs 39.0% (90% CI, 35%-43%) (P = .06) with nab-paclitaxel days 1 and 8 every 3 weeks. The pCR rates for nab-paclitaxel schedules in subgroups were only significantly different for TNBC (60.4% vs 50.0%; P = .06). Grade 3 to 4 toxic effects did not differ with or without denosumab. Nonhematologic toxic effects of grade 3 to 4 were higher with nab-paclitaxel weekly (33.7% vs 24.1%; P = .004).
Conclusions and relevance: In this randomized clinical trial, denosumab added to anthracycline/taxane-based NACT did not improve pCR rates. Nab-paclitaxel at a dosage of 125 mg/m2 weekly significantly increased the pCR rate compared with the days 1 and 8, every-3-weeks schedule overall and in TNBC, but generated higher toxicity.
Trial registration: ClinicalTrials.gov Identifier: NCT02682693.
Conflict of interest statement
Figures

References
-
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) . Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 2015;386(10001):1353-1361. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

