P2RX7 Enhances Tumor Control by CD8+ T Cells in Adoptive Cell Therapy
- PMID: 35588154
- PMCID: PMC9250641
- DOI: 10.1158/2326-6066.CIR-21-0691
P2RX7 Enhances Tumor Control by CD8+ T Cells in Adoptive Cell Therapy
Abstract
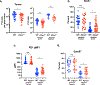
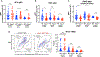
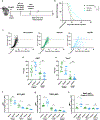
Expression of the purinergic receptor P2RX7 by CD8+ T cells promotes the generation of memory populations following acute infections. However, data suggest that P2RX7 may limit the efficacy of antitumor responses. Herein, we show that P2RX7 is beneficial for optimal melanoma control in a mouse CD8+ T-cell adoptive transfer model. Tumor-specific P2rx7-/- CD8+ T cells exhibited impaired mitochondrial maintenance and function but did not display signs of overt exhaustion early in the antitumor response. However, as the tumor burden increased, the relative frequency of P2RX7-deficient CD8+ T cells declined within the tumor; this correlated with reduced proliferation, increased apoptosis, and mitochondrial dysfunction. Extending these studies, we found that the transient in vitro stimulation of P2RX7 using the ATP analogue BzATP led to enhanced B16 melanoma control by CD8+ T cells. These findings are in keeping with the concept that extracellular ATP (eATP) sensing by P2RX7 on CD8+ T cells is required for their ability to efficiently eliminate tumors by promoting mitochondrial fitness and underscore the potential for P2RX7 stimulation as a novel therapeutic treatment to enhance tumor immunotherapy.
©2022 American Association for Cancer Research.
Conflict of interest statement
Figures




References
-
- Di Virgilio F, et al., The P2X7 Receptor in Infection and Inflammation. Immunity, 2017. 47(1): p. 15–31. - PubMed
-
- Ghiringhelli F, et al., Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med, 2009. 15(10): p. 1170–8. - PubMed
-
- Adinolfi E, et al., Accelerated tumor progression in mice lacking the ATP receptor P2X7. Cancer Res, 2015. 75(4): p. 635–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

