The Impact of the Priority Review Voucher on Research and Development for Tropical Diseases
- PMID: 35588350
- PMCID: PMC9217899
- DOI: 10.1007/s40290-022-00427-x
The Impact of the Priority Review Voucher on Research and Development for Tropical Diseases
Erratum in
-
Correction to: The Impact of the Priority Review Voucher on Research and Development for Tropical Diseases.Pharmaceut Med. 2022 Dec;36(6):405. doi: 10.1007/s40290-022-00440-0. Pharmaceut Med. 2022. PMID: 36344869 Free PMC article. No abstract available.
Abstract
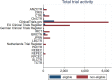
Background: In 2007, the priority review voucher (PRV) was implemented in the US to incentivize research and development (R&D) for tropical diseases. The PRV is issued by the US FDA and grants a quicker review to manufacturers upon successful development of a product for a disease eligible for the program.
Objective: The objective of this analysis was to assess whether the PRV has incentivized R&D (measured as clinical trial activity) for the intended tropical diseases.
Method: We used a difference-in-difference-in-differences (DDD) strategy by exploiting variation in its implementation across diseases and registries around the world. Clinical trials were retrieved from the World Health Organization International Clinical Trials Registry Platform for the years 2005-2019.
Results: We found a positive, but not statistically significant, effect of the PRV on stimulating R&D activity. Delayed effects of the policy could not be found.
Conclusion: Our findings, which were robust across a series of robustness tests, suggest that the PRV program is not associated with a trigger in innovation for neglected diseases and therefore should not be considered as a stand-alone solution. It should be supplemented with other government measures to incentivize R&D activity. To increase the value of the program, we recommend that the PRV only be awarded to novel products and not to products that have already been licensed outside the US. Doing so would restrict the number of vouchers awarded and slow down their ongoing market depreciation. Finally, we propose that product sponsors be required to submit an access plan for PRV-awarded products.
© 2022. The Author(s).
Conflict of interest statement
Céline Aerts, Eliana Barrenho, Marisa Miraldo, and Elisa Sicuri declare no conflicts of interest.
Figures

References
-
- Gaffney A, Mezher M, Brennan Z. Regulatory Explainer: Everything You Need to Know About FDA’s Priority Review Vouchers. 2019. https://www.raps.org/regulatory-focus/news-articles/2017/12/regulatory-e.... Accessed 15 Apr 2022.
-
- United States Government Accountability Office. Drug Development. FDA’s Priority Review Voucher Programs. 2020. https://www.gao.gov/products/gao-20-251. Accessed 15 Apr 2022.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

