New twists to the ALTernative endings at telomeres
- PMID: 35588569
- PMCID: PMC9675980
- DOI: 10.1016/j.dnarep.2022.103342
New twists to the ALTernative endings at telomeres
Abstract
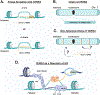
Activation of a telomere maintenance mechanism is key to achieving replicative immortality. Alternative Lengthening of Telomeres (ALT) is a telomerase-independent pathway that hijacks the homologous recombination pathways to elongate telomeres. Commitment to ALT is often associated with several hallmarks including long telomeres of heterogenous lengths, mutations in histone H3.3 or the ATRX/DAXX histone chaperone complex, and incorporation of non-canonical telomere sequences. The consequences of these genetic and epigenetic changes include enhanced replication stress and the presence of transcriptionally permissive chromatin, which can result in replication-associated DNA damage. Here, we detail the molecular mechanisms that are critical to repairing DNA damage at ALT telomeres, including the BLM Helicase, which acts at several steps in the ALT process. Furthermore, we discuss the emerging findings related to the telomere-associated RNA, TERRA, and its roles in maintaining telomeric integrity. Finally, we review new evidence for therapeutic interventions for ALT-positive cancers which are rooted in understanding the molecular underpinnings of this process.
Keywords: ALT; Cancer; Chromatin; TERRA; Telomere.
Copyright © 2022. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest
The authors have no conflicts of interest to declare.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

