Safety and immunogenicity of the Rotavac and Rotasiil rotavirus vaccines administered in an interchangeable dosing schedule among healthy Indian infants: a multicentre, open-label, randomised, controlled, phase 4, non-inferiority trial
- PMID: 35588754
- PMCID: PMC9464301
- DOI: 10.1016/S1473-3099(22)00161-X
Safety and immunogenicity of the Rotavac and Rotasiil rotavirus vaccines administered in an interchangeable dosing schedule among healthy Indian infants: a multicentre, open-label, randomised, controlled, phase 4, non-inferiority trial
Abstract
Background: Rotavirus is the leading cause of severe dehydrating gastroenteritis among children younger than 5 years in low-income and middle-income countries. Two vaccines-Rotavac and Rotasiil-are used in routine immunisation in India. The safety and immunogenicity of these vaccines administered in a mixed regimen is not documented. We therefore aimed to compare the safety and seroresponse of recipients of a mixed regimen versus a single regimen.
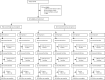
Methods: We did a multicentre, open-label, randomised, controlled, phase 4, non-inferiority trial at two sites in India. We recruited healthy infants aged 6-8 weeks. Infants with systemic disorders, weight-for-height Z scores of less than minus three SDs, or a history of persistent diarrhoea were excluded. Eligible infants were randomly allocated to six groups in equal numbers to receive either the single vaccine regimen (ie, Rotavac-Rotavac-Rotavac [group 1] or Rotasiil-Rotasiil-Rotasiil [group 2]) or the mixed vaccine regimen (ie, Rotavac-Rotasiil-Rotavac [group 3], Rotasiil-Rotavac-Rotasiil [group 4], Rotavac-Rotasiil-Rotasiil [group 5], or Rotasiil-Rotavac-Rotavac [group 6]). Randomisation was done using an online software by site in blocks of at least 12. The primary outcome was seroresponse to rotavirus vaccine, measured using rotavirus-specific serum IgA antibodies 4 weeks after the third dose. The seroresponse rates were compared between recipients of the four mixed vaccine regimens (consisting of various combinations of Rotavac and Rotasiil) with recipients of the single vaccine regimens (consisting of Rotavac or Rotasiil only for all three doses). The non-inferiority margin was set at 10%. Safety follow-ups were done for the duration of study participation. This trial was registered with the Clinical Trials Registry India, number CTRI/2018/08/015317.
Findings: Between March 25, 2019, and Jan 15, 2020, a total of 1979 eligible infants were randomly assigned to receive a single vaccine regimen (n=659; 329 in group 1 and 330 in group 2) or a mixed vaccine regimen (n=1320; 329 each in groups 3 and 4, and 331 each in groups 5 and 6). All eligible participants received the first dose, 1925 (97·3%) of 1979 received the second dose, and 1894 (95·7%) received all three doses of vaccine. 1852 (93·6%) of 1979 participants completed the follow-up. The immunogenicity analysis consisted of 1839 infants (1238 [67·3%] in the mixed vaccine regimen and 601 [32·7%] in the single vaccine regimen; 13 samples were insufficient in quantity) who completed vaccination and provided post-vaccination sera. The seroresponse rate in the mixed vaccine regimen group (33·5% [95% CI 30·9-36·2]) was non-inferior compared with the single vaccine regimen group (29·6% [26·1-33·4]); the seroresponse rate difference was 3·9% (95% CI -0·7 to 8·3). The proportion of participants with any type of solicited adverse events was 90·9% (95% CI 88·4-93·0) in the single vaccine regimen group and 91·1% (89·5-92·6) in the mixed vaccine regimen group. No vaccine-related serious adverse events or intussusception were reported during the study.
Interpretation: Rotavac and Rotasiil can be safely used in an interchangeable manner for routine immunisation since the seroresponse was non-inferior in the mixed vaccine regimen compared with the single vaccine regimen. These results allow for flexibility in administering the vaccines, helping to overcome vaccine shortages and supply chain issues, and targeting migrant populations easily.
Funding: Ministry of Health and Family Welfare, Government of India.
Translation: For the Hindi translation of the abstract see Supplementary Materials section.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures
Comment in
-
Vaccine pragmatism in the 21st century.Lancet Infect Dis. 2022 Aug;22(8):1097-1098. doi: 10.1016/S1473-3099(22)00181-5. Epub 2022 May 16. Lancet Infect Dis. 2022. PMID: 35588756 Free PMC article. No abstract available.
References
-
- Rodriguez WJ, Kim HW, Brandt CD, et al. Longitudinal study of rotavirus infection and gastroenteritis in families served by a pediatric medical practice: clinical and epidemiologic observations. Pediatr Infect Dis J. 1987;6:170–176. - PubMed
-
- John J, Sarkar R, Muliyil J, Bhandari N, Bhan MK, Kang G. Rotavirus gastroenteritis in India, 2011–2013: revised estimates of disease burden and potential impact of vaccines. Vaccine. 2014;32(suppl 1):A5–A9. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

