PKC regulation of ion channels: The involvement of PIP2
- PMID: 35588786
- PMCID: PMC9198471
- DOI: 10.1016/j.jbc.2022.102035
PKC regulation of ion channels: The involvement of PIP2
Abstract
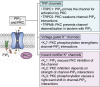
Ion channels are integral membrane proteins whose gating has been increasingly shown to depend on the presence of the low-abundance membrane phospholipid, phosphatidylinositol (4,5) bisphosphate. The expression and function of ion channels is tightly regulated via protein phosphorylation by specific kinases, including various PKC isoforms. Several channels have further been shown to be regulated by PKC through altered surface expression, probability of channel opening, shifts in voltage dependence of their activation, or changes in inactivation or desensitization. In this review, we survey the impact of phosphorylation of various ion channels by PKC isoforms and examine the dependence of phosphorylated ion channels on phosphatidylinositol (4,5) bisphosphate as a mechanistic endpoint to control channel gating.
Keywords: PKC; inwardly rectifying potassium channels; ion channel, phosphorylation; phosphatidylinositol (4,5) bisphosphate; transient receptor potential channels; voltage-gated potassium channel.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

