Mitochondrial calcium uniporter stabilization preserves energetic homeostasis during Complex I impairment
- PMID: 35589699
- PMCID: PMC9120069
- DOI: 10.1038/s41467-022-30236-4
Mitochondrial calcium uniporter stabilization preserves energetic homeostasis during Complex I impairment
Erratum in
-
Author Correction: Mitochondrial calcium uniporter stabilization preserves energetic homeostasis during Complex I impairment.Nat Commun. 2022 Jun 20;13(1):3532. doi: 10.1038/s41467-022-31304-5. Nat Commun. 2022. PMID: 35725853 Free PMC article. No abstract available.
Abstract
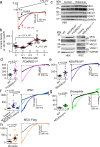
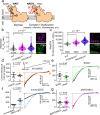
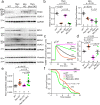
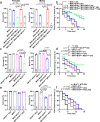
Calcium entering mitochondria potently stimulates ATP synthesis. Increases in calcium preserve energy synthesis in cardiomyopathies caused by mitochondrial dysfunction, and occur due to enhanced activity of the mitochondrial calcium uniporter channel. The signaling mechanism that mediates this compensatory increase remains unknown. Here, we find that increases in the uniporter are due to impairment in Complex I of the electron transport chain. In normal physiology, Complex I promotes uniporter degradation via an interaction with the uniporter pore-forming subunit, a process we term Complex I-induced protein turnover. When Complex I dysfunction ensues, contact with the uniporter is inhibited, preventing degradation, and leading to a build-up in functional channels. Preventing uniporter activity leads to early demise in Complex I-deficient animals. Conversely, enhancing uniporter stability rescues survival and function in Complex I deficiency. Taken together, our data identify a fundamental pathway producing compensatory increases in calcium influx during Complex I impairment.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Territo PR, Mootha VK, French SA, Balaban RS. Ca(2+) activation of heart mitochondrial oxidative phosphorylation: role of the F(0)/F(1)-ATPase. Am. J. Physiol. Cell Physiol. 2000;278:C423–C435. - PubMed
-
- Aydin J, et al. Increased mitochondrial Ca2+ and decreased sarcoplasmic reticulum Ca2+ in mitochondrial myopathy. Hum. Mol. Genet. 2009;18:278–288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

