Intermolecular 2+2 imine-olefin photocycloadditions enabled by Cu(I)-alkene MLCT
- PMID: 35589714
- PMCID: PMC9120151
- DOI: 10.1038/s41467-022-30393-6
Intermolecular 2+2 imine-olefin photocycloadditions enabled by Cu(I)-alkene MLCT
Abstract
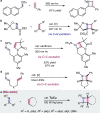
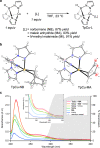
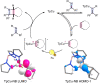
2 + 2 Photocycloadditions are idealized, convergent construction approaches of 4-membered heterocyclic rings, including azetidines. However, methods of direct excitation are limited by the unfavorable photophysical properties of imines and electronically unbiased alkenes. Here, we report copper-catalyzed photocycloadditions of non-conjugated imines and alkenes to produce a variety of substituted azetidines. Design principles allow this base metal-catalyzed method to achieve 2 + 2 imine-olefin photocycloaddition via selective alkene activation through a coordination-MLCT pathway supported by combined experimental and computational mechanistic studies.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Mehra V, Lumb I, Anand A, Kumar V. Recent advances in synthetic facets of immensely reactive azetidines. RSC Adv. 2017;7:45763–45783. doi: 10.1039/C7RA08884A. - DOI

