Integrated analysis of an in vivo model of intra-nasal exposure to instilled air pollutants reveals cell-type specific responses in the placenta
- PMID: 35589747
- PMCID: PMC9119931
- DOI: 10.1038/s41598-022-12340-z
Integrated analysis of an in vivo model of intra-nasal exposure to instilled air pollutants reveals cell-type specific responses in the placenta
Abstract
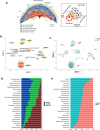
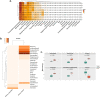
The placenta is a heterogeneous organ whose development involves complex interactions of trophoblasts with decidual, vascular, and immune cells at the fetal-maternal interface. It maintains a critical balance between maternal and fetal homeostasis. Placental dysfunction can lead to adverse pregnancy outcomes including intra-uterine growth restriction, pre-eclampsia, or pre-term birth. Exposure to environmental pollutants contributes to the development of placental abnormalities, with poorly understood molecular underpinning. Here we used a mouse (C57BL/6) model of environmental pollutant exposure by administration of a particulate matter (SRM1649b at 300 μg/day/mouse) suspension intra-nasally beginning 2 months before conception and during gestation, in comparison to saline-exposed controls. Placental transcriptomes, at day 19 of gestation, were determined using bulk RNA-seq from whole placentas of exposed (n = 4) and control (n = 4) animals and scRNAseq of three distinct placental layers, followed by flow cytometry analysis of the placental immune cell landscape. Our results indicate a reduction in vascular placental cells, especially cells responsible for structural integrity, and increase in trophoblast proliferation in animals exposed to particulate matter. Pollution-induced inflammation was also evident, especially in the decidual layer. These data indicate that environmental exposure to air pollutants triggers changes in the placental cellular composition, mediating adverse pregnancy outcomes.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Periconceptional exposure to lopinavir, but not darunavir, impairs decidualization: a potential mechanism leading to poor birth outcomes in HIV-positive pregnancies.Hum Reprod. 2020 Aug 1;35(8):1781-1796. doi: 10.1093/humrep/deaa151. Hum Reprod. 2020. PMID: 32712670 Free PMC article.
-
Something old, something new: digital quantification of uterine vascular remodelling and trophoblast plugging in historical collections provides new insight into adaptation of the utero-placental circulation.Hum Reprod. 2021 Feb 18;36(3):571-586. doi: 10.1093/humrep/deaa303. Hum Reprod. 2021. PMID: 33600565
-
Decidual and placental NOD1 is associated with inflammation in normal and preeclamptic pregnancies.Placenta. 2021 Feb;105:23-31. doi: 10.1016/j.placenta.2021.01.014. Epub 2021 Jan 20. Placenta. 2021. PMID: 33529885
-
An integrated model of preeclampsia: a multifaceted syndrome of the maternal cardiovascular-placental-fetal array.Am J Obstet Gynecol. 2022 Feb;226(2S):S963-S972. doi: 10.1016/j.ajog.2020.10.023. Epub 2021 Mar 9. Am J Obstet Gynecol. 2022. PMID: 33712272 Review.
-
Failure of physiological transformation and spiral artery atherosis: their roles in preeclampsia.Am J Obstet Gynecol. 2022 Feb;226(2S):S895-S906. doi: 10.1016/j.ajog.2020.09.026. Epub 2020 Sep 21. Am J Obstet Gynecol. 2022. PMID: 32971013 Review.
Cited by
-
Placental single cell transcriptomics: Opportunities for endocrine disrupting chemical toxicology.Mol Cell Endocrinol. 2023 Dec 1;578:112066. doi: 10.1016/j.mce.2023.112066. Epub 2023 Sep 9. Mol Cell Endocrinol. 2023. PMID: 37690473 Free PMC article.
-
Exploring the association between atmospheric pollutants and preterm birth risk in a river valley city.Front Public Health. 2024 Jul 25;12:1415028. doi: 10.3389/fpubh.2024.1415028. eCollection 2024. Front Public Health. 2024. PMID: 39118970 Free PMC article.
-
Relationship between exposure to air pollutants in the first trimester and spontaneous abortion in pregnant women in the river valley city.Sci Rep. 2024 Nov 11;14(1):27609. doi: 10.1038/s41598-024-76181-8. Sci Rep. 2024. PMID: 39528500 Free PMC article.
-
Single-cell RNA sequencing reveals placental response under environmental stress.Nat Commun. 2024 Aug 2;15(1):6549. doi: 10.1038/s41467-024-50914-9. Nat Commun. 2024. PMID: 39095385 Free PMC article.
-
The association of short-term increases in ambient PM2.5 and temperature exposures with stillbirth: racial/ethnic disparities among Medicaid recipients.Am J Epidemiol. 2024 Oct 7;193(10):1372-1383. doi: 10.1093/aje/kwae083. Am J Epidemiol. 2024. PMID: 38770979 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

