Label-free complete absorption microscopy using second generation photoacoustic remote sensing
- PMID: 35589763
- PMCID: PMC9120477
- DOI: 10.1038/s41598-022-11235-3
Label-free complete absorption microscopy using second generation photoacoustic remote sensing
Abstract
In the past decades, absorption modalities have emerged as powerful tools for label-free functional and structural imaging of cells and tissues. Many biomolecules present unique absorption spectra providing chromophore-specific information on properties such as chemical bonding, and sample composition. As chromophores absorb photons the absorbed energy is emitted as photons (radiative relaxation) or converted to heat and under specific conditions pressure (non-radiative relaxation). Modalities like fluorescence microscopy may capture radiative relaxation to provide contrast, while modalities like photoacoustic microscopy may leverage non-radiative heat and pressures. Here we show an all-optical non-contact total-absorption photoacoustic remote sensing (TA-PARS) microscope, which can capture both radiative and non-radiative absorption effects in a single acquisition. The TA-PARS yields an absorption metric proposed as the quantum efficiency ratio (QER), which visualizes a biomolecule's proportional radiative and non-radiative absorption response. The TA-PARS provides label-free visualization of a range of biomolecules enabling convincing analogues to traditional histochemical staining of tissues, effectively providing label-free Hematoxylin and Eosin (H&E)-like visualizations. These findings establish an effective all-optical non-contact total-absorption microscope for label-free inspection of biological materials.
© 2022. The Author(s).
Conflict of interest statement
Authors Benjamin Ecclestone, Kevan Bell, Deepak Dinakaran, John R. Mackey, and Parsin Haji Reza, all have financial interests in IllumiSonics which has provided funding to the PhotoMedicine Labs. Author Sarah Sparkes does not have any competing interests.
Figures



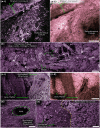
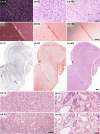
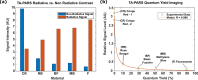

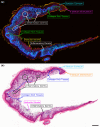

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

