Structure-guided affinity maturation of a novel human antibody targeting the SARS-CoV-2 nucleocapsid protein
- PMID: 35589780
- PMCID: PMC9118815
- DOI: 10.1038/s41598-022-12242-0
Structure-guided affinity maturation of a novel human antibody targeting the SARS-CoV-2 nucleocapsid protein
Abstract
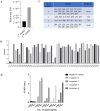
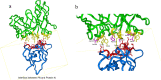
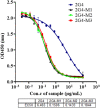
The continuous mutation of SARS-CoV-2 has presented enormous challenges to global pandemic prevention and control. Recent studies have shown evidence that the genome sequence of SARS-CoV-2 nucleocapsid proteins is relatively conserved, and their biological functions are being confirmed. There is increasing evidence that the N protein will not only provide a specific diagnostic marker but also become an effective treatment target. In this study, 2G4, which specifically recognizes the N protein, was identified by screening a human phage display library. Based on the computer-guided homology modelling and molecular docking method used, the 3-D structures for the 2G4 scFv fragment (VH-linker-VL structure, with (G4S)3 as the linker peptide in the model), SARS-CoV-2 N protein and its complex were modelled and optimized with a suitable force field. The binding mode and key residues of the 2G4 and N protein interaction were predicted, and three mutant antibodies (named 2G4-M1, 2G4-M2 and 2G4-M3) with higher affinity were designed theoretically. Using directed point mutant technology, the three mutant antibodies were prepared, and their affinity was tested. Their affinity constants of approximately 0.19 nM (2G4-M1), 0.019 nM (2G4-M2) and 0.075 nM (2G4-M3) were at least one order of magnitude lower than that of the parent antibody (3 nM; 2G4, parent antibody), as determined using a biolayer interferometry (BLI) assay. It is expected that high-affinity candidates will be used for diagnosis and even as potential therapeutic drugs for the SARS-CoV-2 pandemic.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Screening and affinity optimization of single domain antibody targeting the SARS-CoV-2 nucleocapsid protein.PeerJ. 2024 Aug 30;12:e17846. doi: 10.7717/peerj.17846. eCollection 2024. PeerJ. 2024. PMID: 39224822 Free PMC article.
-
Generation and utility of a single-chain fragment variable monoclonal antibody platform against a baculovirus expressed recombinant receptor binding domain of SARS-CoV-2 spike protein.Mol Immunol. 2022 Jan;141:287-296. doi: 10.1016/j.molimm.2021.12.006. Epub 2021 Dec 10. Mol Immunol. 2022. PMID: 34915268 Free PMC article.
-
Biopanning of specific peptide for SARS-CoV-2 nucleocapsid protein and enzyme-linked immunosorbent assay-based antigen assay.Anal Chim Acta. 2023 Jul 11;1264:341300. doi: 10.1016/j.aca.2023.341300. Epub 2023 Apr 29. Anal Chim Acta. 2023. PMID: 37230729 Free PMC article.
-
Antibody Fragments for On-Site Testing of Cannabinoids Generated via in Vitro Affinity Maturation.Biol Pharm Bull. 2017;40(2):174-181. doi: 10.1248/bpb.b16-00669. Biol Pharm Bull. 2017. PMID: 28154257
-
Targeting SARS-CoV2 Spike Protein Receptor Binding Domain by Therapeutic Antibodies.Biomed Pharmacother. 2020 Oct;130:110559. doi: 10.1016/j.biopha.2020.110559. Epub 2020 Aug 1. Biomed Pharmacother. 2020. PMID: 32768882 Free PMC article. Review.
Cited by
-
Identifying highly active anti-CCR4 CAR T cells for the treatment of T-cell lymphoma.Blood Adv. 2023 Jul 25;7(14):3416-3430. doi: 10.1182/bloodadvances.2022008327. Blood Adv. 2023. PMID: 37058474 Free PMC article.
-
A novel MARV glycoprotein-specific antibody with potentials of broad-spectrum neutralization to filovirus.Elife. 2024 Mar 25;12:RP91181. doi: 10.7554/eLife.91181. Elife. 2024. PMID: 38526940 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

