Phosphoinositides as membrane organizers
- PMID: 35589852
- PMCID: PMC9117997
- DOI: 10.1038/s41580-022-00490-x
Phosphoinositides as membrane organizers
Abstract
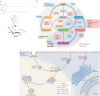
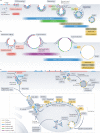
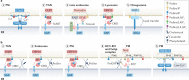
Phosphoinositides are signalling lipids derived from phosphatidylinositol, a ubiquitous phospholipid in the cytoplasmic leaflet of eukaryotic membranes. Initially discovered for their roles in cell signalling, phosphoinositides are now widely recognized as key integrators of membrane dynamics that broadly impact on all aspects of cell physiology and on disease. The past decade has witnessed a vast expansion of our knowledge of phosphoinositide biology. On the endocytic and exocytic routes, phosphoinositides direct the inward and outward flow of membrane as vesicular traffic is coupled to the conversion of phosphoinositides. Moreover, recent findings on the roles of phosphoinositides in autophagy and the endolysosomal system challenge our view of lysosome biology. The non-vesicular exchange of lipids, ions and metabolites at membrane contact sites in between organelles has also been found to depend on phosphoinositides. Here we review our current understanding of how phosphoinositides shape and direct membrane dynamics to impact on cell physiology, and provide an overview of emerging concepts in phosphoinositide regulation.
© 2022. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

