OsSCL30 overexpression reduces the tolerance of rice seedlings to low temperature, drought and salt
- PMID: 35589923
- PMCID: PMC9120446
- DOI: 10.1038/s41598-022-12438-4
OsSCL30 overexpression reduces the tolerance of rice seedlings to low temperature, drought and salt
Abstract
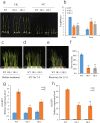
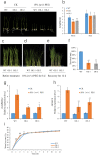
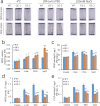
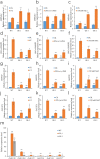
Rice is one of the main food crops for the world population. Various abiotic stresses, such as low temperature, drought, and high salinity, affect rice during the entire growth period, determining its yield and quality, and even leading to plant death. In this study, by constructing overexpression vectors D-163 + 1300:OsSCL30 and D-163 + 1300-AcGFP:OsSCL30-GFP, the mechanism of action of OsSCL30 in various abiotic stresses was explored. Bioinformatics analysis showed that OsSCL30 was located on the chromosome 12 of rice Nipponbare, belonging to the plant-specific SCL subfamily of the SR protein family. The 1500 bp section upstream of the open reading frame start site contains stress-related cis-acting elements such as ABRE, MYC, and MYB. Under normal conditions, the expression of OsSCL30 was higher in leaves and leaf sheaths. The results of reverse transcription polymerase chain reaction showed that the expression of OsSCL30 decreased after low temperature, drought and salt treatment. In root cells OsSCL30 was localized in the nuclei. The results of the rice seedling tolerance and recovery tests showed that overexpression of OsSCL30 diminished the resistance to low temperature, drought and salt stresses in transgenic rice and resulted in larger accumulation of reactive oxygen species. This study is of great significance for exploring the response mechanisms of SR proteins under abiotic stresses.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

