Tyrosine O-sulfation proteoforms affect HIV-1 monoclonal antibody potency
- PMID: 35589938
- PMCID: PMC9120178
- DOI: 10.1038/s41598-022-12423-x
Tyrosine O-sulfation proteoforms affect HIV-1 monoclonal antibody potency
Abstract
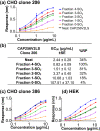
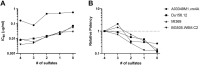
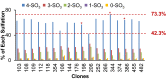
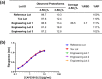
CAP256V2LS, a broadly neutralizing monoclonal antibody (bNAb), is being pursued as a promising drug for HIV-1 prevention. The total level of tyrosine-O-sulfation, a post-translational modification, was known to play a key role for antibody biological activity. More importantly, here wedescribe for the first time the significance of the tyrosine-O-sulfation proteoforms. We developed a hydrophobic interaction chromatography (HIC) method to separate and quantify different sulfation proteoforms, which led to the direct functionality assessment of tyrosine-sulfated species. The fully sulfated (4-SO3) proteoform demonstrated the highest in vitro relative antigen binding potency and neutralization efficiency against a panel of HIV-1 viruses. Interestingly, highly variable levels of 4-SO3 were produced by different clonal CHO cell lines, which helped the bNAb process development towards production of a highly potent CAP256V2LS clinical product with high 4-SO3 proteoform. This study presents powerful insight for any biotherapeutic protein development where sulfation may play an important role in product efficacy.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

