Exploring the Reasons Behind the Substantial Discontinuation Rate Among Patients Taking CT-P13 in a Large Tertiary Hospital in Western Switzerland: A Retrospective Cohort Study Using Routinely Collected Medical Data
- PMID: 35590047
- PMCID: PMC9392673
- DOI: 10.1007/s40801-022-00299-2
Exploring the Reasons Behind the Substantial Discontinuation Rate Among Patients Taking CT-P13 in a Large Tertiary Hospital in Western Switzerland: A Retrospective Cohort Study Using Routinely Collected Medical Data
Abstract
Background: CT-P13 is an infliximab biosimilar that was granted market authorization in Switzerland in 2016. Despite the growing literature supporting the equivalence of CT-P13 compared with originator infliximab regarding the efficacy, safety, and immunogenicity and the undeniable cost-saving opportunities, CT-P13 remains widely underused in Switzerland.
Objective: Leaving aside the phenomenon of a low initiation rate, this study aimed to explore the reasons behind the high discontinuation rate observed among the patients taking CT-P13 in a large tertiary hospital in Western Switzerland.
Methods: We performed a retrospective cohort study using routinely collected data. Patients were eligible if they received originator infliximab or CT-P13 between September 2017 and December 2020. They were included if they had received at least two CT-P13 infusions during the same period. Patients were excluded if the follow-up was incomplete prior to or 6 months after their first CT-P13 infusion and if they had an oncological main diagnosis. Primary outcomes were the reasons for treatment discontinuation.
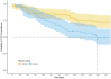
Results: One hundred and fifty-six patients were included and classified into two groups: switchers who were treated with originator infliximab and were switched to CT-P13 (n = 85, 54%) and initiators who did not receive originator infliximab prior to CT-P13 treatment (n = 71, 46%). Included patients belonged to three different groups of diagnosis: gastroenterological (67, 43%), rheumatological (61, 39%), and immunological (28, 18%). Twenty-three (27%) switchers and 35 (49%) initiators discontinued CT-P13 after 12 months. Main reasons for CT-P13 discontinuation were lack of efficacy (n = 21, 36%) and secondary loss of response (n = 16, 28%); however, objective assessments were not available. Initiators' probability to discontinue CT-P13 at 12 months was significantly higher than switchers' (p < 0.01).
Conclusions: Lack of efficacy and secondary loss of response were the main reasons for the high CT-P13 discontinuation rate observed in a large tertiary hospital in Western Switzerland. Lack of active training and coordination among healthcare professionals and little education in patients may have exacerbated patients' subjective complaints and increased the CT-P13 discontinuation rate.
© 2022. The Author(s).
Conflict of interest statement
MK, JCD, JM, and FS declare that they have no conflicts of interest.
Figures

Similar articles
-
Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial.Lancet. 2017 Jun 10;389(10086):2304-2316. doi: 10.1016/S0140-6736(17)30068-5. Epub 2017 May 11. Lancet. 2017. PMID: 28502609 Clinical Trial.
-
Serum concentrations after switching from originator infliximab to the biosimilar CT-P13 in patients with quiescent inflammatory bowel disease (SECURE): an open-label, multicentre, phase 4 non-inferiority trial.Lancet Gastroenterol Hepatol. 2018 Jun;3(6):404-412. doi: 10.1016/S2468-1253(18)30082-7. Epub 2018 Mar 30. Lancet Gastroenterol Hepatol. 2018. PMID: 29606564 Clinical Trial.
-
Efficacy and tolerability of initiating, or switching to, infliximab biosimilar CT-P13 in inflammatory bowel disease (IBD): a large single-centre experience.Scand J Gastroenterol. 2018 Jun;53(6):700-707. doi: 10.1080/00365521.2018.1464203. Epub 2018 Apr 24. Scand J Gastroenterol. 2018. PMID: 29687730
-
Drug Discontinuation in Studies Including a Switch From an Originator to a Biosimilar Monoclonal Antibody: A Systematic Literature Review.Clin Ther. 2019 Jan;41(1):155-173.e13. doi: 10.1016/j.clinthera.2018.11.002. Epub 2018 Dec 11. Clin Ther. 2019. PMID: 30551802
-
CT-P13 in the treatment of rheumatoid arthritis.Expert Rev Clin Immunol. 2017 Jul;13(7):653-666. doi: 10.1080/1744666X.2017.1337510. Expert Rev Clin Immunol. 2017. PMID: 28571501 Review.
Cited by
-
Impact of switching from the originator adalimumab to a biosimilar: a retrospective cohort study.BMC Immunol. 2025 Jul 3;26(1):44. doi: 10.1186/s12865-025-00693-9. BMC Immunol. 2025. PMID: 40610896 Free PMC article.
-
Does the introduction of an infliximab biosimilar always result in savings for hospitals? A descriptive study using real-world data.Health Econ Rev. 2024 Apr 29;14(1):31. doi: 10.1186/s13561-024-00507-5. Health Econ Rev. 2024. PMID: 38683413 Free PMC article.
-
Current Expertise, Opinions, and Attitude toward TNF-⍺ Antagonist Biosimilars among Physicians: A Self-Administered Online Survey in Western Switzerland.Healthcare (Basel). 2022 Oct 28;10(11):2152. doi: 10.3390/healthcare10112152. Healthcare (Basel). 2022. PMID: 36360497 Free PMC article.
-
Factors associated with the uptake of biosimilars for breast cancer treatment from the perspectives of physicians and patients-Evidence from China.Front Pharmacol. 2023 Jan 12;13:1044798. doi: 10.3389/fphar.2022.1044798. eCollection 2022. Front Pharmacol. 2023. PMID: 36712662 Free PMC article.
References
-
- US Food and Drug Administration. Biological product definitions. 2020. https://www.fda.gov/media/108557/download. Accessed 6 Feb 2020
-
- Otto ESA, Schrader U. Rapid growth in biopharma: challenges and opportunities. 2014. https://www.mckinsey.com/~/media/McKinsey/Industries/Healthcare%20System.... Accessed 6 Feb 2020
LinkOut - more resources
Full Text Sources

