Candesartan Protects Against Cadmium-Induced Hepatorenal Syndrome by Affecting Nrf2, NF-κB, Bax/Bcl-2/Cyt-C, and Ang II/Ang 1-7 Signals
- PMID: 35590119
- PMCID: PMC9931870
- DOI: 10.1007/s12011-022-03286-4
Candesartan Protects Against Cadmium-Induced Hepatorenal Syndrome by Affecting Nrf2, NF-κB, Bax/Bcl-2/Cyt-C, and Ang II/Ang 1-7 Signals
Retraction in
-
Retraction Note: Candesartan Protects Against Cadmium-Induced Hepatorenal Syndrome by Affecting Nrf2, NF-κB, Bax/Bcl-2/Cyt-C, and Ang II/Ang 1-7 Signals.Biol Trace Elem Res. 2025 May;203(5):2933. doi: 10.1007/s12011-024-04347-6. Biol Trace Elem Res. 2025. PMID: 39145864 Free PMC article. No abstract available.
Abstract
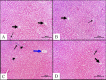
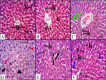
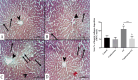
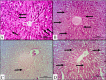
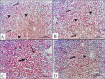
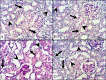
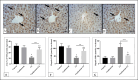
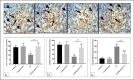
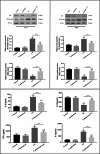
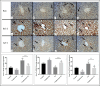
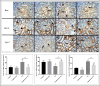
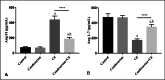
Cadmium (Cd) is a serious pollutant in the environment. Candesartan is an angiotensin II (Ang II) receptor antagonist with promising diverse health benefits. The current study is planned to investigate the hepatorenal protective effects of candesartan against Cd-induced hepatic and renal intoxication. Our results demonstrated that candesartan effectively attenuated Cd-induced hepatorenal intoxication, as evidenced by improving hepatic and renal function biomarkers. Besides, candesartan reversed hepatic and renal histopathological abrasions induced by Cd toxicity. Candesartan antioxidant effect was mediated by Nrf2 activation. Also, candesartan suppressed hepatorenal inflammation by modulating NF-κB/IκB. Moreover, candesartan attenuated Cd hepatorenal apoptosis by upregulating Bcl-2 and downregulating Bax and Cyt-C proteins. Interestingly, these effects are suggested to be an outcome of modulating of Ang II/Ang 1-7 signal. Overall, our findings revealed that candesartan could attenuate Cd-induced hepatorenal intoxication through modulation of Nrf2, NF-κB/IκB, Bax/Bcl-2/Cyt-c, and Ang II/Ang 1-7 signaling pathways.
Keywords: Ang II/Ang 1–7; Bax/Bcl-2/Cyt-C; Cadmium; Candesartan; NF-κB; Nrf2.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

