Risk factors of fracture following curettage for bone giant cell tumors of the extremities
- PMID: 35590280
- PMCID: PMC9118605
- DOI: 10.1186/s12891-022-05447-x
Risk factors of fracture following curettage for bone giant cell tumors of the extremities
Abstract
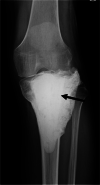
Background: Following curettage of giant cell tumor of bone (GCTB), it is common to fill the cavity with polymethylmethacrylate (PMMA) bone cement, bone allograft, or artificial bone to maintain bone strength; however, there is a 2-14% risk of postoperative fractures. We conducted this retrospective study to clarify the risk factors for fractures after curettage for GCTB of the extremities.
Methods: This study included 284 patients with GCTBs of the extremities who underwent curettage at our institutions between 1980 and 2018 after excluding patients whose cavities were not filled with anything or who had additional plate fixation. The tumor cavity was filled with PMMA bone cement alone (n = 124), PMMA bone cement and bone allograft (n = 81), bone allograft alone (n = 63), or hydroxyapatite graft alone (n = 16).
Results: Fractures after curettage occurred in 10 (3.5%) patients, and the median time from the curettage to fracture was 3.5 months (interquartile range [IQR], 1.8-8.3 months). The median postoperative follow-up period was 86.5 months (IQR, 50.3-118.8 months). On univariate analysis, patients who had GCTB of the proximal or distal femur (1-year fracture-free survival, 92.5%; 95% confidence interval [CI]: 85.8-96.2) presented a higher risk for postoperative fracture than those who had GCTB at another site (100%; p = 0.0005). Patients with a pathological fracture at presentation (1-year fracture-free survival, 88.2%; 95% CI: 63.2-97.0) presented a higher risk for postoperative fracture than those without a pathological fracture at presentation (97.8%; 95% CI: 95.1-99.0; p = 0.048). Patients who received bone grafting (1-year fracture-free survival, 99.4%; 95% CI: 95.7-99.9) had a lower risk of postoperative fracture than those who did not receive bone grafting (94.4%; 95% CI: 88.7-97.3; p = 0.003).
Conclusions: For GCTBs of the femur, especially those with pathological fracture at presentation, bone grafting after curettage is recommended to reduce the risk of postoperative fracture. Additional plate fixation should be considered when curettage and cement filling without bone grafting are performed in patients with GCTB of the femur. This should be specially performed for those patients with a pathological fracture at presentation.
Keywords: Bone grafting; Cement; Curettage; Denosumab; Fracture; Giant cell tumor of bone.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures








References
-
- Flanagan AM, Larousserie F, O’Donnell PG, Yoshida A. World Health Organ Classif Tumours Editorial Board. WHO classification of tumours. 5. Soft Tissue and Bone Tumours. Lyon: IARC; 2020. Giant cell tumour of bone; pp. 440–446.
-
- van der Heijden L, van de Sande MAJ, Heineken AC, Fiocco M, Nelissen RGHH, Dijkstra PDS. Mid-term outcome after curettage with polymethylmethacrylate for giant cell tumor around the knee: higher risk of radiographic osteoarthritis? J Bone Joint Surg Am. 2013;95:e159. doi: 10.2106/JBJS.M.00066. - DOI - PubMed
-
- van der Heijden L, van der Geest ICM, Schreuder HWB, van de Sande MAJ, Dijkstra PDS. Liquid nitrogen or phenolization for giant cell tumor of bone?: a comparative cohort study of various standard treatments at two tertiary referral centers. J Bone Joint Surg Am. 2014;96:e35. doi: 10.2106/JBJS.M.00516. - DOI - PubMed
-
- Uglialoro AD, Maceroli M, Beebe KS, Benevenia J, Patterson FR. Distal femur defects reconstructed with polymethylmethacrylate and internal fixation devices: a biomechanical study. Orthopedics. 2009;32 orthosupersite.com/view.asp?rID=41918. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

