Influence of Femtosecond Laser Modification on Biomechanical and Biofunctional Behavior of Porous Titanium Substrates
- PMID: 35591307
- PMCID: PMC9099494
- DOI: 10.3390/ma15092969
Influence of Femtosecond Laser Modification on Biomechanical and Biofunctional Behavior of Porous Titanium Substrates
Abstract
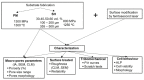
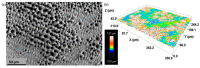
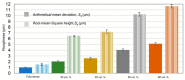
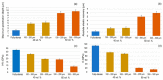
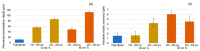
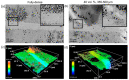
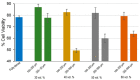
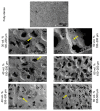
Bone resorption and inadequate osseointegration are considered the main problems of titanium implants. In this investigation, the texture and surface roughness of porous titanium samples obtained by the space holder technique were modified with a femtosecond Yb-doped fiber laser. Different percentages of porosity (30, 40, 50, and 60 vol.%) and particle range size (100-200 and 355-500 μm) were compared with fully-dense samples obtained by conventional powder metallurgy. After femtosecond laser treatment the formation of a rough surface with micro-columns and micro-holes occurred for all the studied substrates. The surface was covered by ripples over the micro-metric structures. This work evaluates both the influence of the macro-pores inherent to the spacer particles, as well as the micro-columns and the texture generated with the laser, on the wettability of the surface, the cell behavior (adhesion and proliferation of osteoblasts), micro-hardness (instrumented micro-indentation test, P-h curves) and scratch resistance. The titanium sample with 30 vol.% and a pore range size of 100-200 μm was the best candidate for the replacement of small damaged cortical bone tissues, based on its better biomechanical (stiffness and yield strength) and biofunctional balance (bone in-growth and in vitro osseointegration).
Keywords: cellular behavior; femtosecond laser; instrumented micro-indentation; porous titanium; scratch test; surface modification; wettability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Approach to the Fatigue and Cellular Behavior of Superficially Modified Porous Titanium Dental Implants.Materials (Basel). 2022 May 30;15(11):3903. doi: 10.3390/ma15113903. Materials (Basel). 2022. PMID: 35683200 Free PMC article.
-
Biofunctional and Tribomechanical Behavior of Porous Titanium Substrates Coated with a Bioactive Glass Bilayer (45S5-1393).ACS Appl Mater Interfaces. 2020 Jul 8;12(27):30170-30180. doi: 10.1021/acsami.0c07318. Epub 2020 Jun 25. ACS Appl Mater Interfaces. 2020. PMID: 32530265
-
Nanotextured porous titanium scaffolds by argon ion irradiation: Toward conformal nanopatterning and improved implant osseointegration.J Biomed Mater Res A. 2023 Dec;111(12):1850-1865. doi: 10.1002/jbm.a.37582. Epub 2023 Jun 19. J Biomed Mater Res A. 2023. PMID: 37334879
-
Improved Corrosion Behavior and Biocompatibility of Porous Titanium Samples Coated with Bioactive Chitosan-Based Nanocomposites.Materials (Basel). 2021 Oct 22;14(21):6322. doi: 10.3390/ma14216322. Materials (Basel). 2021. PMID: 34771848 Free PMC article.
-
Surface modification of zirconia dental implants by laser texturing.Lasers Med Sci. 2022 Feb;37(1):77-93. doi: 10.1007/s10103-021-03475-y. Epub 2022 Jan 13. Lasers Med Sci. 2022. PMID: 35022871 Review.
Cited by
-
Fabrication of Au Nanostructured Thin Film via Femtosecond Laser Glass Texturing for Enhanced Glucose Sensing.ACS Omega. 2025 Feb 28;10(9):9165-9176. doi: 10.1021/acsomega.4c09270. eCollection 2025 Mar 11. ACS Omega. 2025. PMID: 40092760 Free PMC article.
-
Stability, biomechanics and biocompatibility analysis following different preparation strategies of hierarchical zeolite coatings on titanium alloy surfaces.Front Bioeng Biotechnol. 2023 Dec 22;11:1337709. doi: 10.3389/fbioe.2023.1337709. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 38188487 Free PMC article.
References
-
- Khorasani A.M., Goldberg M., Doeven E.H., Littlefair G. Titanium in Biomedical Applications—Properties and Fabrication: A Review. J. Biomater. Tissue Eng. 2015;5:593–619. doi: 10.1166/jbt.2015.1361. - DOI
-
- Chen L.-Y., Cui Y.-W., Zhang L.-C. Recent Development in Beta Titanium Alloys for Biomedical Applications. Metals. 2020;10:1139. doi: 10.3390/met10091139. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

