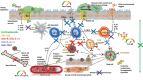
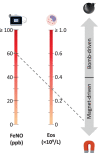
Sub-stratification of type-2 high airway disease for therapeutic decision-making: A 'bomb' (blood eosinophils) meets 'magnet' (FeNO) framework
- PMID: 35591794
- PMCID: PMC9541235
- DOI: 10.1111/resp.14294
Sub-stratification of type-2 high airway disease for therapeutic decision-making: A 'bomb' (blood eosinophils) meets 'magnet' (FeNO) framework
Keywords: airway markers; asthma; eosinophils; inflammation; nitric oxide.
Conflict of interest statement
Simon Couillard received non‐restricted research grants from Sanofi‐Genzyme, the Quebec Respiratory Health Research Network and the Association Pulmonaire du Québec, outside the submitted work; received speaker honoraria from GlaxoSmithKline, Sanofi‐Regeneron and AstraZeneca, outside the submitted work; and is on the advisory board of Biometry Inc, outside the submitted work. In the last 5 years, Ian D. Pavord has received speaker's honoraria for speaking at sponsored meetings from AstraZeneca, Boehringer Ingelheim, Aerocrine AB, Almirall, Novartis, Teva, Chiesi, Sanofi/Regeneron, Menarini and GSK, and payments for organizing educational events from AstraZeneca, GSK, Sanofi/Regeneron and Teva. He has received honoraria for attending advisory panels with Genentech, Sanofi/Regeneron, AstraZeneca, Boehringer Ingelheim, GSK, Novartis, Teva, Merck, Circassia, Chiesi and Knopp, and payments to support FDA approval meetings from GSK. He has received sponsorship to attend international scientific meetings from Boehringer Ingelheim, GSK, AstraZeneca, Teva and Chiesi. He has received grants from Chiesi and Sanofi Genzyme. He is co‐patent holder of the rights to the Leicester Cough Questionnaire and has received payments for its use in clinical trials from Merck, Bayer and Insmed. During 2014–2015, he was an expert witness for a patent dispute involving AstraZeneca and Teva. Liam G. Heaney has received grant funding, participated in advisory boards and given lectures at meetings supported by Amgen, AstraZeneca, Boehringer Ingelheim, Circassia, Hoffmann la Roche, GlaxoSmithKline, Novartis, Theravance, Evelo Biosciences, Sanofi and Teva. He has received grants from MedImmune, Novartis UK, Roche/Genentech Inc, Glaxo Smith Kline, Amgen, Genentech/Hoffman la Roche, Astra Zeneca, MedImmune, Glaxo Smith Kline, Aerocrine and Vitalograph. He has received sponsorship for attending international scientific meetings from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK and Napp Pharmaceuticals. He has also taken part in asthma clinical trials sponsored by AstraZeneca, Boehringer Ingelheim, Hoffmann la Roche and GlaxoSmithKline for which his institution received remuneration. He is the Academic Lead for the Medical Research Council Stratified Medicine UK Consortium in Severe Asthma which involves industrial partnerships with a number of pharmaceutical companies including Amgen, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Hoffmann la Roche and Janssen. Nayia Petousi is supported from the NIHR Oxford BRC. She has received grants from the University of Oxford and NIHR research capability funding from Oxford University Hospitals outside the submitted work. She has received personal fees from Astra Zeneca, outside the submitted work. Timothy S. C. Hinks has received grants from the Wellcome Trust, The Guardians of the Beit Fellowship, Pfizer Inc., Kymab Ltd, Sensyne Health, University of Oxford and from the NIHR Oxford BRC, outside the submitted work. He has received personal fees from Astra Zeneca, TEVA, Omniprex and Peer Voice, outside the submitted work.
Figures

Similar articles
-
Inflammatory Markers to Inform Treatment of Asthma With Biologicals: FeNO Versus Blood Eosinophils.J Allergy Clin Immunol Pract. 2023 Apr;11(4):1221-1223. doi: 10.1016/j.jaip.2023.02.001. J Allergy Clin Immunol Pract. 2023. PMID: 37030927 No abstract available.
-
Derivation of a prototype asthma attack risk scale centred on blood eosinophils and exhaled nitric oxide.Thorax. 2022 Feb;77(2):199-202. doi: 10.1136/thoraxjnl-2021-217325. Epub 2021 Aug 6. Thorax. 2022. PMID: 34362839 Free PMC article.
-
Clinical and inflammatory features of asthma with dissociation between fractional exhaled nitric oxide and eosinophils in induced sputum.J Asthma. 2016 Jun;53(5):459-64. doi: 10.3109/02770903.2015.1116086. Epub 2016 Jan 19. J Asthma. 2016. PMID: 26785727
-
Utility of serum periostin in combination with exhaled nitric oxide in the management of asthma.Allergol Int. 2017 Jul;66(3):404-410. doi: 10.1016/j.alit.2017.02.003. Epub 2017 Feb 28. Allergol Int. 2017. PMID: 28256388 Review.
-
Tailoring asthma treatment on eosinophilic markers (exhaled nitric oxide or sputum eosinophils): a systematic review and meta-analysis.Thorax. 2018 Dec;73(12):1110-1119. doi: 10.1136/thoraxjnl-2018-211540. Epub 2018 Jun 1. Thorax. 2018. PMID: 29858277
Cited by
-
Asthma Pathogenesis: Phenotypes, Therapies, and Gaps: Summary of the Aspen Lung Conference 2023.Am J Respir Cell Mol Biol. 2024 Aug;71(2):154-168. doi: 10.1165/rcmb.2024-0082WS. Am J Respir Cell Mol Biol. 2024. PMID: 38635858 Free PMC article. Review.
-
Biologics alter the relationship between exhaled nitric oxide fraction and sputum eosinophils in severe T2-high asthma patients.ERJ Open Res. 2025 Jun 16;11(3):00783-2024. doi: 10.1183/23120541.00783-2024. eCollection 2025 May. ERJ Open Res. 2025. PMID: 40524926 Free PMC article.
-
Comparative efficacy of tezepelumab to mepolizumab, benralizumab, and dupilumab in eosinophilic asthma: A Bayesian network meta-analysis.J Allergy Clin Immunol. 2023 Mar;151(3):747-755. doi: 10.1016/j.jaci.2022.11.021. Epub 2022 Dec 17. J Allergy Clin Immunol. 2023. PMID: 36538979 Free PMC article.
-
Resting state functional connectivity changes following mindfulness-based stress reduction predict improvements in disease control for patients with asthma.Brain Behav Immun. 2024 Jan;115:480-493. doi: 10.1016/j.bbi.2023.10.026. Epub 2023 Nov 2. Brain Behav Immun. 2024. PMID: 37924961 Free PMC article. Clinical Trial.
-
Recovery of Breakthrough Asthma Attacks Treated With Oral Steroids While on Monoclonal Antibody Therapy: Protocol for a Prospective Observational Study (BOOST).JMIR Res Protoc. 2023 Jun 23;12:e46741. doi: 10.2196/46741. JMIR Res Protoc. 2023. PMID: 37351918 Free PMC article.
References
-
- Menzies‐Gow A, Gurnell M, Heaney LG, Corren J, Bel EH, Maspero J, et al. Oral corticosteroid elimination via a personalised reduction algorithm in adults with severe, eosinophilic asthma treated with benralizumab (PONENTE): a multicentre, open‐label, single‐arm study. Lancet Respir Med. 2022;10(1):47–58. 10.1016/S2213-2600(21)00352-0 - DOI - PubMed
-
- ReportsnReports . Asthma Treatment Market (Drug) Worth $23 Billion by 2023. [Cited 16 May 2022]. Available from: https://www.prnewswire.com/news‐releases/asthma‐treatment‐market‐drug‐wo...
-
- Barnes PJ. COUNTERPOINT: will new anti‐eosinophilic drugs be useful in asthma management? No. Chest. 2017;151:17–20. - PubMed
-
- Couillard S, Pavord ID. Biological therapies for asthma. In: James S, editor. Encyclopedia of respiratory medicine. Elsevier, Amsterdam. 2022. p. 411–34.

