Long non-coding RNA LINC01296 promotes progression of oral squamous cell carcinoma through activating the MAPK/ERK signaling pathway via the miR-485-5p/PAK4 axis
- PMID: 35591837
- PMCID: PMC9102572
- DOI: 10.5114/aoms.2019.86805
Long non-coding RNA LINC01296 promotes progression of oral squamous cell carcinoma through activating the MAPK/ERK signaling pathway via the miR-485-5p/PAK4 axis
Abstract
Introduction: Long intergenic non-protein-coding RNA 1296 (LINC01296), a newly identified lncRNA, can function as an oncogenic driver to promote the development of multiple carcinomas. However, the effect of LINC01296 on oral squamous cell carcinoma (OSCC) is still unclear.
Material and methods: We determined the expression and role of LINC01296 in OSCC tissues and cell lines. The cell viability, migration and invasion were determined by MTT, wound healing assay and transwell assay, respectively. Flow cytometry was used for detecting cell cycle and apoptosis. The interaction and association between LINC01296, microRNA-485-5p (miR-485-5p) and p21 (RAC1) activated kinase 4 (PAK4) were analyzed by RNA immunoprecipitation (RIP) and luciferase reporter assays. The xenograft mouse model was established to detect the effect of LINC01296 on OSCC tumor growth.
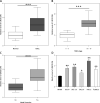
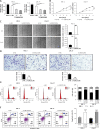
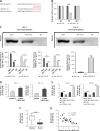
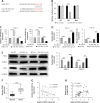
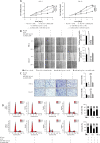
Results: Our study showed that LINC01296 was over-expressed in OSCC tissues and cell lines. The level of LINC01296 was positively correlated with the patient's tumor node metastasis (TNM) stage and nodal invasion. Knockdown of LINC01296 effectively inhibits cell viability, migration and invasion but promotes cell apoptosis in vitro. The in vivo experiment showed that LINC01296 knockdown inhibited OSCC tumor growth. The following analysis indicated that LINC01296 acted as a ceRNA for miR-485-5p, and PAK4 was identified as a direct target of miR-485-5p. Furthermore, we found that the effects of LINC01296 on OSCC progression were through regulating the expression of PAK4/p-MEK/p-ERK via sponging miR-485-5p.
Conclusions: LINC01296 promote the cell cycle, proliferation, migration and invasion, and inhibit apoptosis of OSCC cells through activating the MAPK/ERK signaling pathway via sponging miR-485-5p to regulate PAK4 expression. These results suggested that the LINC01296/miR-485-5p/PAK4 axis was closely associated with OSCC progression. Our study provides a new insight into the molecular pathogenesis of OSCC, and may supply novel biomarkers for diagnosis and therapy of OSCC.
Keywords: LINC01296; MAPK/ERK signaling pathway; PAK4; miR-485-5p; oral squamous cell carcinoma.
Copyright: © 2019 Termedia & Banach.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
MicroRNA-140-5p inhibits the tumorigenesis of oral squamous cell carcinoma by targeting p21-activated kinase 4.Cell Biol Int. 2020 Jan;44(1):145-154. doi: 10.1002/cbin.11213. Epub 2019 Aug 21. Cell Biol Int. 2020. PMID: 31393040
-
Long noncoding RNA LINC01296 regulates the cell proliferation, migration and invasion in neuroblastoma.Metab Brain Dis. 2022 Apr;37(4):1247-1258. doi: 10.1007/s11011-022-00935-4. Epub 2022 Mar 19. Metab Brain Dis. 2022. PMID: 35305236
-
LncRNA HOXA11-AS promotes OSCC progression by sponging miR-98-5p to upregulate YBX2 expression.Biomed Pharmacother. 2020 Jan;121:109623. doi: 10.1016/j.biopha.2019.109623. Epub 2019 Nov 12. Biomed Pharmacother. 2020. PMID: 31731187
-
MicroRNA-31: a pivotal oncogenic factor in oral squamous cell carcinoma.Cell Death Discov. 2022 Mar 29;8(1):140. doi: 10.1038/s41420-022-00948-z. Cell Death Discov. 2022. PMID: 35351880 Free PMC article. Review.
-
The significance of PAK4 in signaling and clinicopathology: A review.Open Life Sci. 2022 Jun 20;17(1):586-598. doi: 10.1515/biol-2022-0064. eCollection 2022. Open Life Sci. 2022. PMID: 35800076 Free PMC article. Review.
Cited by
-
The Prognostic Value and Clinical Significance of lncRNA SNHG5 Expression in Patients with Multiple Malignancies: A Bioinformatic and Meta-analysis.Curr Cancer Drug Targets. 2024;24(12):1286-1297. doi: 10.2174/0115680096282865240111055640. Curr Cancer Drug Targets. 2024. PMID: 38409690
-
Non-coding RNAs in leukemia drug resistance: new perspectives on molecular mechanisms and signaling pathways.Ann Hematol. 2024 May;103(5):1455-1482. doi: 10.1007/s00277-023-05383-3. Epub 2023 Aug 1. Ann Hematol. 2024. PMID: 37526673 Review.
-
LINC01296 promotes cancer stemness traits in oral carcinomas by sponging miR-143.J Dent Sci. 2023 Apr;18(2):814-821. doi: 10.1016/j.jds.2023.01.008. Epub 2023 Jan 23. J Dent Sci. 2023. PMID: 37021272 Free PMC article.
References
-
- Rivera C, Oliveira AK, Costa RAP, De Rossi T, Paes Leme AF. Prognostic biomarkers in oral squamous cell carcinoma: a systematic review. Oral Oncol 2017; 72: 38-47. - PubMed
-
- Ishida K, Tomita H, Nakashima T, et al. . Current mouse models of oral squamous cell carcinoma: genetic and chemically induced models. Oral Oncol 2017; 73: 16-20. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
