Toll-like Interleukin -1 Receptor Regulator (TILRR) Protein, a Major Modulator of Inflammation, is Expressed in Normal Human and Macaque Tissues and PBMCs
- PMID: 35592073
- PMCID: PMC9113122
- DOI: 10.2147/JIR.S357866
Toll-like Interleukin -1 Receptor Regulator (TILRR) Protein, a Major Modulator of Inflammation, is Expressed in Normal Human and Macaque Tissues and PBMCs
Abstract
Purpose: TILRR is a modulator of genes in the NF-κB inflammation pathway. It regulates inflammation-responsive genes, the secretion of inflammatory mediators, and the migration of immune cells. Because inflammation drives the pathogenesis of many infectious and inflammatory diseases, it is important to know the expression of TILRR protein in tissues and cells. This study examined TILRR protein expression in healthy adult human and macaques' tissues and PBMCs (peripheral blood mononuclear cells).
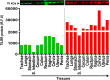
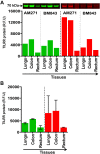
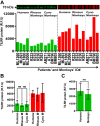
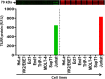
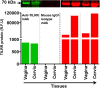
Methods and results: Tissues (trachea, lungs, stomach, small intestine [ileum], cecum, colon, rectum, vagina, cervix, uterus, and penis) and PBMCs from humans and macaques were lysed in RIPA (radioimmunoprecipitation assay) lysis buffer. The TILRR protein was examined by fluorescent Western blot analysis. The relative fluorescence units (rfu) of TILRR protein expression were quantified by Image Studio software (LI-COR). The results showed that adult healthy female (n=1) rectal and cervicovaginal tissues expressed a higher level of TILRR protein than the other tissues (trachea, lungs, stomach, small intestine [ileum], cecum, colon, uterus, and penis) examined. Like humans, the lungs, colon, and rectal tissues of healthy adult female cynomolgus monkeys (Macaca fascicularis) (n=2) expressed the TILRR protein. In addition, PBMCs of healthy adult women (n=4), adult female cynomolgus monkeys (Macaca fascicularis) (n=4), and adult male and female rhesus monkeys (Macaca mulatta) (n=4) showed a similar expression level of TILRR protein (p= 0.2858). TILRR protein was not detected in most of the human cell lines examined, except in Jurkat cells.
Conclusion: Our study for the first time showed that TILRR protein is expressed in healthy adult human and monkey tissues and PBMCs. The TILRR protein in these tissues and PBMCs may play a role in the inflammatory response of these tissues and cells in response to infectious pathogens.
Keywords: PBMCs; TILRR; cynomolgus monkey (Macaca fascicularis); human; inflammation; rhesus monkey (Macaca mulatta); tissues.
© 2022 Kashem et al.
Conflict of interest statement
The authors declare no competing interest.
Figures
Similar articles
-
TILRR (Toll-like Interleukin-1 Receptor Regulator), an Important Modulator of Inflammatory Responsive Genes, is Circulating in the Blood.J Inflamm Res. 2021 Sep 24;14:4927-4943. doi: 10.2147/JIR.S325553. eCollection 2021. J Inflamm Res. 2021. PMID: 34594127 Free PMC article.
-
Toll-like Interleukin 1 Receptor Regulator Is an Important Modulator of Inflammation Responsive Genes.Front Immunol. 2019 Feb 28;10:272. doi: 10.3389/fimmu.2019.00272. eCollection 2019. Front Immunol. 2019. PMID: 30873160 Free PMC article.
-
High level of plasma TILRR protein is associated with faster HIV seroconversion.EBioMedicine. 2022 Apr;78:103955. doi: 10.1016/j.ebiom.2022.103955. Epub 2022 Mar 24. EBioMedicine. 2022. PMID: 35339895 Free PMC article.
-
The Potential Role of FREM1 and Its Isoform TILRR in HIV-1 Acquisition through Mediating Inflammation.Int J Mol Sci. 2021 Jul 22;22(15):7825. doi: 10.3390/ijms22157825. Int J Mol Sci. 2021. PMID: 34360591 Free PMC article. Review.
-
Genetic polymorphisms of drug-metabolizing cytochrome P450 enzymes in cynomolgus and rhesus monkeys and common marmosets in preclinical studies for humans.Biochem Pharmacol. 2018 Jul;153:184-195. doi: 10.1016/j.bcp.2017.12.015. Epub 2017 Dec 23. Biochem Pharmacol. 2018. PMID: 29277691 Review.
References
LinkOut - more resources
Full Text Sources

