Environmental Enrichment and Estrogen Upregulate Beta-Hydroxybutyrate Underlying Functional Improvement
- PMID: 35592114
- PMCID: PMC9113201
- DOI: 10.3389/fnmol.2022.869799
Environmental Enrichment and Estrogen Upregulate Beta-Hydroxybutyrate Underlying Functional Improvement
Abstract
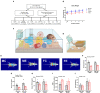
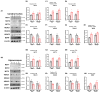
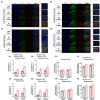
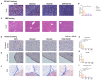
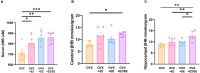
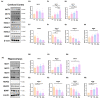
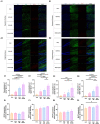
Environmental enrichment (EE) is a promising therapeutic strategy in improving metabolic and neuronal responses, especially due to its non-invasive nature. However, the exact mechanism underlying the sex-differential effects remains unclear. The aim of the current study was to investigate the effects of EE on metabolism, body composition, and behavioral phenotype based on sex. Long-term exposure to EE for 8 weeks induced metabolic changes and fat reduction. In response to the change in metabolism, the level of βHB were influenced by sex and EE possibly in accordance to the phases of estrogen cycle. The expression of β-hydroxybutyrate (βHB)-related genes and proteins such as monocarboxylate transporters, histone deacetylases (HDAC), and brain-derived neurotrophic factor (BDNF) were significantly regulated. In cerebral cortex and hippocampus, EE resulted in a significant increase in the level of βHB and a significant reduction in HDAC, consequently enhancing BDNF expression. Moreover, EE exerted significant effects on motor and cognitive behaviors, indicating a significant functional improvement in female mice under the condition that asserts the influence of estrogen cycle. Using an ovariectomized mice model, the effects of EE and estrogen treatment proved the hypothesis that EE upregulates β-hydroxybutyrate and BDNF underlying functional improvement in female mice. The above findings demonstrate that long-term exposure to EE can possibly alter metabolism by increasing the level of βHB, regulate the expression of βHB-related proteins, and improve behavioral function as reflected by motor and cognitive presentation following the changes in estrogen level. This finding may lead to a marked improvement in metabolism and neuroplasticity by EE and estrogen level.
Keywords: beta-hydroxybutyrate (β-HB); brain derived neurotrophic factor (BDNF); environmental enrichment (EE); estrogen; female; functional improvement; neuroplasticity; sex.
Copyright © 2022 Pyo, Kim, Hwang, Heo, Kim and Cho.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer SK declared a shared affiliation with the authors to the handling editor at the time of review.
Figures






Similar articles
-
Environmental enrichment blocks ethanol-induced locomotor sensitization and decreases BDNF levels in the prefrontal cortex in mice.Addict Biol. 2012 Jul;17(4):736-45. doi: 10.1111/j.1369-1600.2011.00408.x. Epub 2011 Nov 29. Addict Biol. 2012. PMID: 22126132
-
Late-Life Environmental Enrichment Induces Acetylation Events and Nuclear Factor κB-Dependent Regulations in the Hippocampus of Aged Rats Showing Improved Plasticity and Learning.J Neurosci. 2016 Apr 13;36(15):4351-61. doi: 10.1523/JNEUROSCI.3239-15.2016. J Neurosci. 2016. PMID: 27076430 Free PMC article.
-
Sex-Dependent Effects of Environmental Enrichment on Spatial Memory and Brain-Derived Neurotrophic Factor (BDNF) Signaling in a Developmental "Two-Hit" Mouse Model Combining BDNF Haploinsufficiency and Chronic Glucocorticoid Stimulation.Front Behav Neurosci. 2018 Oct 9;12:227. doi: 10.3389/fnbeh.2018.00227. eCollection 2018. Front Behav Neurosci. 2018. PMID: 30356704 Free PMC article.
-
Environmental Enrichment Effects on the Brain-Derived Neurotrophic Factor Expression in Healthy Condition, Alzheimer's Disease, and Other Neurodegenerative Disorders.J Alzheimers Dis. 2022;85(3):975-992. doi: 10.3233/JAD-215193. J Alzheimers Dis. 2022. PMID: 34897089 Review.
-
Differential impact of stress and environmental enrichment on corticolimbic circuits.Pharmacol Biochem Behav. 2020 Oct;197:172993. doi: 10.1016/j.pbb.2020.172993. Epub 2020 Jul 11. Pharmacol Biochem Behav. 2020. PMID: 32659243 Free PMC article. Review.
Cited by
-
Activation of β2-adrenergic receptors prevents AD-type synaptotoxicity via epigenetic mechanisms.Mol Psychiatry. 2023 Nov;28(11):4877-4888. doi: 10.1038/s41380-023-02145-5. Mol Psychiatry. 2023. PMID: 37365243
-
Effects of Sex and Cross-Sex Hormone Treatment on Renal MCT/SMCT Expression Following Prepubertal Gonadectomy.Pharmaceutics. 2025 Feb 14;17(2):252. doi: 10.3390/pharmaceutics17020252. Pharmaceutics. 2025. PMID: 40006619 Free PMC article.
References
-
- Ballestri S., Nascimbeni F., Baldelli E., Marrazzo A., Romagnoli D., Lonardo A. (2017). NAFLD as a sexual dimorphic disease: role of gender and reproductive status in the development and progression of nonalcoholic fatty liver disease and inherent cardiovascular risk. Adv. Ther. 34, 1291–1326. 10.1007/s12325-017-0556-1 - DOI - PMC - PubMed
-
- Benjamin D., Robay D., Hindupur S. K., Pohlmann J., Colombi M., El-Shemerly M. Y., et al. . (2018). Dual inhibition of the lactate transporters MCT1 and MCT4 is synthetic lethal with metformin due to NAD+ depletion in cancer cells. Cell Rep. 25, 3047–3058.e4. 10.1016/j.celrep.2018.11.043 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

