Clinical, Virological and Immunological Subphenotypes in a Cohort of Early Treated HIV-Infected Children
- PMID: 35592310
- PMCID: PMC9111748
- DOI: 10.3389/fimmu.2022.875692
Clinical, Virological and Immunological Subphenotypes in a Cohort of Early Treated HIV-Infected Children
Abstract
Background: Identifying subphenotypes within heterogeneous diseases may have an impact in terms of therapeutic options. In this study, we aim to assess different subphenotypes in children living with human immunodeficiency virus (HIV-1), according to the clinical, virological, and immunological characteristics.
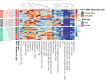
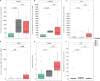
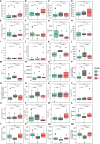
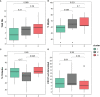
Methods: We collected clinical and sociodemographic data, baseline viral load (VL), CD4 and CD8 count and percentage, age at initiation of ART, HIV DNA reservoir size in peripheral blood mononuclear cells (PBMCs), cell-associated RNA (CA-RNA), ultrasensitive VL, CD4 subsets (T effector CD25+, activated memory cells, Treg cells), humoral-specific HIV response (T-bet B cells), innate response (CD56dim natural killer (NK) cells, NKp46+, perforin), exhaustion markers (PD-1, PD-L1, DNAM), CD8 senescence, and biomarkers for T-lymphocyte thymic output (TREC) and endothelial activation (VCAM). The most informative variables were selected using an unsupervised lasso-type penalty selection for sparse clustering. Hierarchical clustering was performed using Pearson correlation as the distance metric and WARD.D2 as the clustering method. Internal validation was applied to select the best number of clusters. To compare the characteristics among clusters, boxplot and Kruskal Wallis test were assessed.
Results: Three subphenotypes were discovered (cluster1: n=18, 45%; cluster2: n=11, 27.5%; cluster3: n=11, 27.5%). Patients in cluster1 were treated earlier, had higher baseline %CD4, low HIV reservoir size, low western blot score, higher TREC values, and lower VCAM values than the patients in the other clusters. In contrast, cluster3 was the less favorable. Patients were treated later and presented poorer outcomes with lower %CD4, and higher reservoir size, along with a higher percentage of CD8 immunosenescent cells, lower TREC, higher VCAM cytokine, and a higher %CD4 PD-1. Cluster2 was intermediate. Patients were like those of cluster1, but had lower levels of t-bet expression and higher HIV DNA reservoir size.
Conclusions: Three HIV pediatric subphenotypes with different virological and immunological features were identified. The most favorable cluster was characterized by a higher rate of immune reconstitution and a slower disease progression, and the less favorable with more senescence and high reservoir size. In the near future therapeutic interventions for a path of a cure might be guided or supported by the different subphenotypes.
Keywords: HIV; immune signatures; pediatric; perinatal; reservoir; subphenotypes; viral dynamics.
Copyright © 2022 Domínguez-Rodríguez, Tagarro, Foster, Palma, Cotugno, Zicari, Ruggiero, de Rossi, Dalzini, Pahwa, Rinaldi, Nastouli, Marcelin, Dorgham, Sauce, Gartner, Rossi, Giaquinto and Rojo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Check Hayden E. HIV Vaccine Failure Explained? Nat Rev Immunol (2008) 6:930–9. doi: 10.1038/NEWS.2008.1208 - DOI
-
- Parisi SG, Sarmati L, Andreis S, Scaggiante R, Cruciani M, Ferretto R, et al. . Strong and Persistent Correlation Between Baseline and Follow-Up HIV-DNA Levels and Residual Viremia in a Population of Naïve Patients With More Than 4 Years of Effective Antiretroviral Therapy. Clin Microbiol Infect (2015) 21:288.e5–7. doi: 10.1016/j.cmi.2014.10.009 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

