HDAC Inhibition as Potential Therapeutic Strategy to Restore the Deregulated Immune Response in Severe COVID-19
- PMID: 35592335
- PMCID: PMC9111747
- DOI: 10.3389/fimmu.2022.841716
HDAC Inhibition as Potential Therapeutic Strategy to Restore the Deregulated Immune Response in Severe COVID-19
Abstract
The COVID-19 pandemic has had a devastating impact worldwide and has been a great challenge for the scientific community. Vaccines against SARS-CoV-2 are now efficiently lessening COVID-19 mortality, although finding a cure for this infection is still a priority. An unbalanced immune response and the uncontrolled release of proinflammatory cytokines are features of COVID-19 pathophysiology and contribute to disease progression and worsening. Histone deacetylases (HDACs) have gained interest in immunology, as they regulate the innate and adaptative immune response at different levels. Inhibitors of these enzymes have already proven therapeutic potential in cancer and are currently being investigated for the treatment of autoimmune diseases. We thus tested the effects of different HDAC inhibitors, with a focus on a selective HDAC6 inhibitor, on immune and epithelial cells in in vitro models that mimic cells activation after viral infection. Our data indicate that HDAC inhibitors reduce cytokines release by airway epithelial cells, monocytes and macrophages. This anti-inflammatory effect occurs together with the reduction of monocytes activation and T cell exhaustion and with an increase of T cell differentiation towards a T central memory phenotype. Moreover, HDAC inhibitors hinder IFN-I expression and downstream effects in both airway epithelial cells and immune cells, thus potentially counteracting the negative effects promoted in critical COVID-19 patients by the late or persistent IFN-I pathway activation. All these data suggest that an epigenetic therapeutic approach based on HDAC inhibitors represents a promising pharmacological treatment for severe COVID-19 patients.
Keywords: COVID-19; Histone deacetylases; T cell exhaustion; cytokines; immune response; inflammation.
Copyright © 2022 Ripamonti, Spadotto, Pozzi, Stevenazzi, Vergani, Marchini, Sandrone, Bonetti, Mazzarella, Minucci, Steinkühler and Fossati.
Conflict of interest statement
CR, VS, PP, AS, BV, MM, GS, GF, and CS are employees of Italfarmaco. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


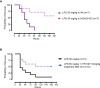

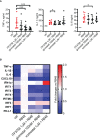



References
-
- Gamage AM, Tan KS, Chan WOY, Liu J, Tan CW, Ong YK, et al. . Infection of Human Nasal Epithelial Cells With SARS-CoV-2 and a 382-Nt Deletion Isolate Lacking ORF8 Reveals Similar Viral Kinetics and Host Transcriptional Profiles. PloS Pathog (2020) 16:e10009130. doi: 10.1371/journal.ppat.1009130 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

