The Effect of Pregabalin on the Minimum Alveolar Concentration of Sevoflurane: A Randomized, Placebo-Controlled, Double-Blind Clinical Trial
- PMID: 35592857
- PMCID: PMC9110662
- DOI: 10.3389/fmed.2022.883181
The Effect of Pregabalin on the Minimum Alveolar Concentration of Sevoflurane: A Randomized, Placebo-Controlled, Double-Blind Clinical Trial
Abstract
Background: Pregabalin is commonly used perioperatively to reduce post-operative pain and opioid consumption and to prevent the development of chronic pain. It has been shown to reduce anesthetic consumption in balanced anesthesia, but studies investigating its effect on the minimum alveolar concentration (MAC) of volatile anesthetics are lacking. The aim of this study was to investigate the effect of two different doses of pregabalin on the MAC of sevoflurane.
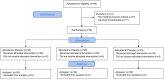
Methods: In a randomized, double-blinded, placebo controlled clinical study, 75 patients were assigned to receive placebo, 300 mg pregabalin, or 150 mg pregabalin, as a capsule 1 h before anesthesia induction with sevoflurane only. After equilibration, the response to skin incision (movement vs. non-movement) was monitored. The MAC was assessed using an up- and down-titration method.
Results: The MAC of sevoflurane was estimated as 2.16% (95% CI, 2.07-2.32%) in the placebo group, 1.44% (95% CI, 1.26-1.70%) in the 300 mg pregabalin group, and 1.81% (95% CI, 1.49-2.13%) in the 150 mg pregabalin group. We therefore report a 33% reduction in the MAC of sevoflurane in the 300 mg pregabalin group as compared to placebo. The MAC of the 150 mg pregabalin group was reduced by 16% as compared to placebo but was not statistically significant.
Conclusions: The administration of 300 mg pregabalin reduced the MAC of sevoflurane by 33%, while the administration of 150 mg pregabalin did not significantly reduce the MAC of sevoflurane. Pregabalin use led to a small reduction in post-operative pain levels but increased side effects in a dose-dependent manner.
Keywords: anesthesia; depth of anesthesia; minimum alveolar concentration (MAC); pregabalin; premedication before anesthesia; sevoflurane.
Copyright © 2022 Müller, Plöchl, Mühlbacher, Graf, Stimpfl and Hamp.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
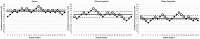
Similar articles
-
The effect of a bolus dose of intravenous lidocaine on the minimum alveolar concentration of sevoflurane: a prospective, randomized, double-blinded, placebo-controlled trial.Anesth Analg. 2013 Aug;117(2):323-8. doi: 10.1213/ANE.0b013e318294820f. Epub 2013 Jun 6. Anesth Analg. 2013. PMID: 23744959 Clinical Trial.
-
Does epidural anesthesia have general anesthetic effects? A prospective, randomized, double-blind, placebo-controlled trial.Anesthesiology. 1999 Dec;91(6):1687-92. doi: 10.1097/00000542-199912000-00021. Anesthesiology. 1999. PMID: 10598611 Clinical Trial.
-
Effect of intravenous S-ketamine on the MAC of sevoflurane: a randomised, placebo-controlled, double-blinded clinical trial.Br J Anaesth. 2018 Dec;121(6):1242-1248. doi: 10.1016/j.bja.2018.08.023. Epub 2018 Oct 15. Br J Anaesth. 2018. PMID: 30442251 Clinical Trial.
-
Patients Undergoing Orthotopic Liver Transplantation Require Lower Concentrations of the Volatile Anesthetic Sevoflurane.Anesth Analg. 2017 Sep;125(3):783-789. doi: 10.1213/ANE.0000000000002250. Anesth Analg. 2017. PMID: 28678075 Clinical Trial.
-
Epidural lidocaine decreases sevoflurane requirement for adequate depth of anesthesia as measured by the Bispectral Index monitor.Anesthesiology. 2001 May;94(5):799-803. doi: 10.1097/00000542-200105000-00018. Anesthesiology. 2001. PMID: 11388531 Clinical Trial.
References
-
- Lyrica . European Medicines Agency. Available online at: https://www.ema.europa.eu/en/medicines/human/EPAR/lyrica#product-informa... (accessed January 11, 2022).
LinkOut - more resources
Full Text Sources

