Origin of M2 Mϕ and its macrophage polarization by TGF-β in a mice intervertebral injury model
- PMID: 35592891
- PMCID: PMC9174651
- DOI: 10.1177/03946320221103792
Origin of M2 Mϕ and its macrophage polarization by TGF-β in a mice intervertebral injury model
Abstract
Introduction: Studies have identified the presence of M1 and M2 macrophages (Mϕ) in injured intervertebral discs (IVDs). However, the origin and polarization-regulatory factor of M2 Mϕ are not fully understood. TGF-β is a regulatory factor for M2 polarization in several tissues. Here, we investigated the source of M2 Mϕ and the role of TGF-β on M2 polarization using a mice disc-puncture injury model.
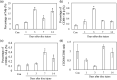
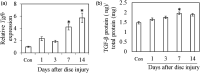
Methods: To investigate the origin of M2 macrophages, 30 GFP chimeric mice were created by bone marrow transplantation. IVDs were obtained from both groups on pre-puncture (control) and post-puncture days 1, 3, 7, and 14 and CD86 (M1 marker)- and CD206 (M2 marker)-positive cells evaluated by flow cytometry (n = 5 at each time point). To investigate the role of TGF-β on M2 polarization, TGF-β inhibitor (SB431542) was also injected on post-puncture days (PPD) 5 and 6 and CD206 expression was evaluated on day 7 by flow cytometry (n = 5) and real time PCR (n = 10).
Results: The proportion of CD86+ Mϕ within the GFP+ population was significantly increased at PPD 1, 3, 7, and 14 compared to control. CD206-positive cells in GFP-populations were significantly increased on PPD 7 and 14. In addition, the percentage of CD206-positive cells was significantly higher in GFP-populations than in GFP+ populations. TGF-β inhibitor reduced CD206-positive cells and Cd206 expression at 7 days after puncture.
Conclusion: Our findings suggest that M2 Mϕ following IVD injury may originate from resident Mϕ. TGF-β is a key factor for M2 polarization of macrophages following IVD injury.
Keywords: Macrophages; TGF-β; resident.
Conflict of interest statement
Figures





Similar articles
-
Reduced TGF-β Expression and CD206-Positive Resident Macrophages in the Intervertebral Discs of Aged Mice.Biomed Res Int. 2021 Jul 12;2021:7988320. doi: 10.1155/2021/7988320. eCollection 2021. Biomed Res Int. 2021. PMID: 34337052 Free PMC article.
-
Changes in Nerve Growth Factor Expression and Macrophage Phenotype Following Intervertebral Disc Injury in Mice.J Orthop Res. 2019 Aug;37(8):1798-1804. doi: 10.1002/jor.24308. Epub 2019 Apr 24. J Orthop Res. 2019. PMID: 30977543
-
TGF-β regulates nerve growth factor expression in a mouse intervertebral disc injury model.BMC Musculoskelet Disord. 2021 Jul 23;22(1):634. doi: 10.1186/s12891-021-04509-w. BMC Musculoskelet Disord. 2021. PMID: 34301215 Free PMC article.
-
The Role of M1/M2 Macrophage Polarization in Rheumatoid Arthritis Synovitis.Front Immunol. 2022 May 19;13:867260. doi: 10.3389/fimmu.2022.867260. eCollection 2022. Front Immunol. 2022. PMID: 35663975 Free PMC article. Review.
-
[The role of macrophage polarization and interaction with renal tubular epithelial cells in ischemia-reperfusion induced acute kidney injury].Sheng Li Xue Bao. 2022 Feb 25;74(1):28-38. Sheng Li Xue Bao. 2022. PMID: 35199123 Review. Chinese.
Cited by
-
Nucleus Pulposus Cells Induce M2 Polarization of RAW264.7 via CX3CL1/CX3CR1 Pathway and M2 Macrophages Promote Proliferation and Anabolism of Nucleus Pulposus Cells.Stem Cells Int. 2023 Feb 20;2023:6400162. doi: 10.1155/2023/6400162. eCollection 2023. Stem Cells Int. 2023. Retraction in: Stem Cells Int. 2024 Jan 24;2024:9869743. doi: 10.1155/2024/9869743. PMID: 37274023 Free PMC article. Retracted.
-
Role of macrophage in intervertebral disc degeneration.Bone Res. 2025 Jan 23;13(1):15. doi: 10.1038/s41413-024-00397-7. Bone Res. 2025. PMID: 39848963 Free PMC article. Review.
-
Immune exposure: how macrophages interact with the nucleus pulposus.Front Immunol. 2023 Apr 14;14:1155746. doi: 10.3389/fimmu.2023.1155746. eCollection 2023. Front Immunol. 2023. PMID: 37122738 Free PMC article. Review.
-
Stiffness regulates dendritic cell and macrophage subtype development and increased stiffness induces a tumor-associated macrophage phenotype in cancer co-cultures.Front Immunol. 2024 Aug 15;15:1434030. doi: 10.3389/fimmu.2024.1434030. eCollection 2024. Front Immunol. 2024. PMID: 39211033 Free PMC article.
-
M2 macrophages activate the IL-10/JAK2/STAT3 pathway to induce pathological microangiogenesis in the nucleus pulposus exacerbating intervertebral disc degeneration.J Orthop Surg Res. 2025 May 28;20(1):532. doi: 10.1186/s13018-025-05962-2. J Orthop Surg Res. 2025. PMID: 40426248 Free PMC article.
References
-
- Takada T, Nishida K, Doita M, et al. (2004) Interleukin-6 production is upregulated by interaction between disc tissue and macrophages. Spine (Phila Pa 1976) 29: 1089–1092. discussion 1093. - PubMed
-
- Takada T, Nishida K, Maeno K, et al. (2012) Intervertebral disc and macrophage interaction induces mechanical hyperalgesia and cytokine production in a herniated disc model in rats. Arthritis & Rheumatology 64: 2601–2610. - PubMed
-
- Miyagi M, Uchida K, Takano S, et al. (2018) Macrophage-derived inflammatory cytokines regulate growth factors and pain-related molecules in mice with intervertebral disc injury. Journal of Orthopaedic Research 36: 2274–2279. - PubMed
-
- Miyagi M, Uchida K, Takano S, et al. (2020) Role of CD14-positive cells in inflammatory cytokine and pain-related molecule expression in human degenerated intervertebral discs. Journal of Orthopaedic Research 39: 1755–1762. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

