NNK from tobacco smoking enhances pancreatic cancer cell stemness and chemoresistance by creating a β2AR-Akt feedback loop that activates autophagy
- PMID: 35593085
- PMCID: PMC9348597
- DOI: 10.1002/1878-0261.13230
NNK from tobacco smoking enhances pancreatic cancer cell stemness and chemoresistance by creating a β2AR-Akt feedback loop that activates autophagy
Abstract
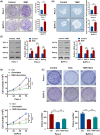
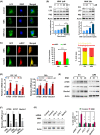
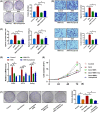
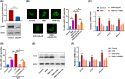
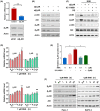
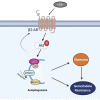
Low responsiveness to chemotherapy is an important cause of poor prognosis in pancreatic cancer. Smoking is a high-risk factor for pancreatic cancer and cancer resistance to gemcitabine; however, the underlying mechanisms remain unclear. 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) is the main metabolite of tobacco burning and has been shown to be associated with cancer development and chemoresistance. However, in pancreatic cancer, its mechanism remains poorly understood. In this study, we found that NNK promoted stemness and gemcitabine resistance in pancreatic cancer cell lines. Moreover, NNK increased autophagy and elevated the expression levels of the autophagy-related markers autophagy-related gene 5 (ATG5), autophagy-related gene 7 (ATG7), and Beclin1. Furthermore, the results showed that NNK-promoted stemness and gemcitabine resistance was partially dependent on the role of NNK in cell autophagy, which is mediated by the β2-adrenergic receptor (β2AR)-Akt axis. Finally, we proved that NNK intervention could not only activate β2AR, but also increase its expression, making β2AR and Akt form a feedback loop. Overall, these findings show that the NNK-induced β2AR-Akt feedback loop promotes stemness and gemcitabine resistance in pancreatic cancer cells.
Keywords: NNK; autophagy; chemoresistance; pancreatic cancer; stemness.
© 2022 The Authors. Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

