Astrocyte Cell Surface Antigen 2 and Other Potential Cell Surface Markers of Enteric glia in the Mouse Colon
- PMID: 35593118
- PMCID: PMC9125112
- DOI: 10.1177/17590914221083203
Astrocyte Cell Surface Antigen 2 and Other Potential Cell Surface Markers of Enteric glia in the Mouse Colon
Abstract
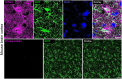
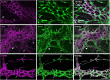
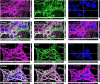
Enteric glia regulate gut functions in health and disease through diverse interactions with neurons and immune cells. Intracellular localization of traditional markers of enteric glia such as GFAP, s100b, and Sox10 makes them incompatible for studies that require antigen localization at the cell surface. Thus, new tools are needed for probing the heterogeneous roles of enteric glia at the protein, cell, and functional levels. Here we selected several cell surface antigens including Astrocyte Cell Surface Marker 2 (ACSA2), Cluster of differentiation 9 (CD9), lysophosphatidic acid receptor 1 (LPAR1), and Proteolipid protein 1 (PLP1) as potential markers of enteric glia. We tested their specificity for enteric glia using published single-cell/-nuclei and glia-specific translating mRNA enriched transcriptome datasets, immunolabeling, and flow cytometry. The data show that ACSA2 is a specific marker of mucosal and myenteric glia while other markers are suitable for identifying all subpopulations of enteric glia (LPAR1), glia and immune cells (CD9), or are not suitable for cell-surface labeling (PLP1). These new tools will be useful for future work focused on understanding specific glial functions in health and disease.Summary StatementThis study identifies astrocyte cell surface antigen 2 as a novel marker of myenteric glia in the intestine. This, in combination with other markers identified in this study, could be used for selective targeting of enteric glia.
Keywords: ACSA2; CD9; LPAR1; PLP1; enteric nervous system.
Conflict of interest statement
Figures



Similar articles
-
Functional Intraregional and Interregional Heterogeneity between Myenteric Glial Cells of the Colon and Duodenum in Mice.J Neurosci. 2022 Nov 16;42(46):8694-8708. doi: 10.1523/JNEUROSCI.2379-20.2022. Epub 2022 Nov 1. J Neurosci. 2022. PMID: 36319118 Free PMC article.
-
Astrocyte-like glia in the peripheral nervous system: an immunohistochemical study of enteric glia.J Neurosci. 1983 Nov;3(11):2206-18. doi: 10.1523/JNEUROSCI.03-11-02206.1983. J Neurosci. 1983. PMID: 6138397 Free PMC article.
-
Enteric Glia Regulate Gastrointestinal Motility but Are Not Required for Maintenance of the Epithelium in Mice.Gastroenterology. 2017 Oct;153(4):1068-1081.e7. doi: 10.1053/j.gastro.2017.07.002. Epub 2017 Jul 13. Gastroenterology. 2017. PMID: 28711628 Free PMC article.
-
Enteric Glia: S100, GFAP, and Beyond.Anat Rec (Hoboken). 2019 Aug;302(8):1333-1344. doi: 10.1002/ar.24128. Epub 2019 Apr 29. Anat Rec (Hoboken). 2019. PMID: 30951262 Review.
-
Enteric glial cells and their role in the intestinal epithelial barrier.World J Gastroenterol. 2014 Aug 28;20(32):11273-80. doi: 10.3748/wjg.v20.i32.11273. World J Gastroenterol. 2014. PMID: 25170211 Free PMC article. Review.
Cited by
-
SOX10-Mediated Regulation of Enteric Glial Phenotype in vitro and its Relevance for Neuroinflammatory Disorders.J Mol Neurosci. 2025 Feb 21;75(1):26. doi: 10.1007/s12031-025-02321-y. J Mol Neurosci. 2025. PMID: 39982575 Free PMC article.
-
Functional analysis of antigen presentation by enteric glial cells during intestinal inflammation.Glia. 2025 Feb;73(2):291-308. doi: 10.1002/glia.24632. Epub 2024 Nov 4. Glia. 2025. PMID: 39495092 Free PMC article.
References
-
- Ahmadzai, M. M., McClain, J. L., Dharshika, C., Seguella L., Giancola, F., De Giorgio, R., Gulbransen, B. D. (2022). LPAR1 regulates enteric nervous system function through glial signaling and contributes to chronic intestinal pseudo-obstruction. The Journal of Clinical Investigation, 132(4): e149464. 10.1172/JCI149464 - DOI - PMC - PubMed
-
- Batiuk M. Y., de Vin F., Duque S. I., Li C., Saito T., Saido T., Fiers M., Belgard T. G., Holt M. G. (2017). An immunoaffinity-based method for isolating ultrapure adult astrocytes based on ATP1B2 targeting by the ACSA-2 antibody. Journal of Biological Chemistry, 292(21), 8874–8891. 10.1074/jbc.M116.765313 - DOI - PMC - PubMed
-
- Batiuk M. Y., Martirosyan A., Wahis J., de Vin F., Marneffe C., Kusserow C., Koeppen J., Viana J. F., Oliveira J. F., Voet T., Ponting C. P., Belgard T. G., Holt M. G. (2020). Identification of region-specific astrocyte subtypes at single cell resolution. Nature Communications, 11(1), 1220. 10.1038/s41467-019-14198-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

